Abstract
There is a vast amount of evidence from psychological studies that the amount of parent–child interaction affects the development of children's verbal skills and knowledge. However, despite the vast amount of literature, brain structural development associated with the amount of parent–child interaction has never been investigated. In the present human study, we used voxel-based morphometry to measure regional gray matter density (rGMD) and examined cross-sectional correlations between the amount of time spent with parents and rGMD among 127 boys and 135 girls. We also assessed correlations between the amount of time spent with parents and longitudinal changes that occurred a few years later among 106 boys and 102 girls. After correcting for confounding factors, we found negative effects of spending time with parents on rGMD in areas in the bilateral superior temporal gyrus (STG) via cross-sectional analyses as well as in the contingent areas of the right STG. We also confirmed positive effects of spending time with parents on the Verbal Comprehension score in cross-sectional and longitudinal analyses. rGMD in partly overlapping or contingent areas of the right STG was negatively correlated with age and the Verbal Comprehension score in cross-sectional analyses. Subsequent analyses revealed verbal parent–child interactions have similar effects on Verbal Comprehension scores and rGMD in the right STG in both cross-sectional and longitudinal analyses. These findings indicate that parent–child interactions affect the right STG, which may be associated with verbal skills.
Keywords: children, development, gray matter density, parent, verbal
Introduction
Many cross-sectional and longitudinal studies have reported the importance of parent–child interactions in children's verbal development. Numerous studies have shown positive effects of parental verbal stimulation and responsivity (Bing, 1963; Clarke-Stewart, 1973; Bradley and Caldwell, 1976; Fewell and Deutscher, 2002), higher amount of parent–child interaction or verbal parent–child interaction (Jones, 1972; Bradley and Caldwell, 1976; McCartney, 1984), and types of parent–child communicative style (Bee et al., 1969; Henderson, 1970; Tulkin and Kagan, 1972; Ramey et al., 1979) as well as negative effects of the parental employment status that prevents more parent–child interaction (Parcel and Menaghan, 1990; Han et al., 2001; Ruhm, 2004) on verbal skills and knowledge during infancy and later developmental phases.
Previous neuroimaging studies have revealed the gray matter structural characteristics of children exposed to parental verbal abuse (De Bellis et al., 2002a) and of mistreated children and adolescents with post-traumatic stress disorder (Tomoda et al., 2011) via cross-sectional studies. Exposure to maltreatment and abuse is also related to children's verbal skills (Friederici et al., 2009), and both are clinically important. However, no neuroimaging studies have revealed (1) the effect of amount of parent–child interaction on neural systems, (2) after controlling for the affective tone of child–parent relationship in nonclinical samples, (3) from the perspective of associations with children's verbal skills' development (4) in longitudinal studies. This is despite the vast amount of traditional focus on effects of parent–child interaction on verbal skills. Children's verbal skills are important not only from the intellectual perspective but also from the emotional (Funk and Ruppert, 1984) and social (Milligan et al., 2007) perspective, therefore revealing that the neural mechanisms underlying the effects of parent–child relationship is essential.
The bilateral superior temporal gyrus (STG) and left inferior frontal gyrus (IFG) are considered to be important for the development of verbal skills in children via social interaction, such as parent–child interaction. The bilateral STG plays key roles in Verbal Comprehension (Friederici et al., 2009) and in the comprehension of nonliteral or nonverbal aspects of communicative information (Desimone, 1991; McGuire et al., 1996). Furthermore, maltreatment, abuse, and low socioeconomic status are related to children's lower verbal skills (Culp et al., 1991), and they alter the gray matter structures of the bilateral STG (De Bellis et al., 2002a; Tomoda et al., 2011; Krishnadas et al., 2013). Finally, verbal skills show negative correlations with the amount of gray matter in the bilateral STG (Menary et al., 2013). On the other hand, the left IFG has been shown to be critical for a wide range of verbal cognitions (Vigneau et al., 2006) and verbal development (Ramsden et al., 2011). Therefore, we hypothesized that the amount of parent–child interaction, which affects children's verbal development, would also result in rGM changes in bilateral STG and the left IFG.
The present study aimed to test this hypothesis using cross-sectional and longitudinal analyses of brain structures in nonclinical children after controlling for the affective tone of the parent–child relationship.
Materials and Methods
Subjects.
All subjects were healthy Japanese children, and the details related to their initial recruitment (preexperiment) were described previously (Taki et al., 2010). In brief, we collected brain magnetic resonance (MR) images from 290 native Japanese subjects (145 boys and 145 girls; age range, 5.6–18.4 years) who did not have any history of malignant tumors or head traumas involving loss of consciousness. Further, based on the self-report, children with a history of epilepsy, impaired color vision, diagnosis of developmental disorders, routine visits to a hospital because of illness, congenital disorders, or routine medications (except daily drugs, such as cold or anti-allergy medications) were excluded during the recruitment processes. We did not use specific diagnostic tools, although the second author is a radiologist and thoroughly checked the T1-weighted structural images for unfound neurological diseases. We stipulated that only right-handed children could participate in the study in an advertisement used for subject recruitment and also confirmed that all subjects were right-handed using the self-report questionnaire, the Edinburgh Handedness Inventory (Oldfield, 1971). As per the Declaration of Helsinki (1991), written informed consent was obtained from each subject and his/her parent before MR scanning after a full explanation of the purpose and procedures of the study was provided. Approval for these experiments was obtained from the Institutional Review Board of Tohoku University. A few years after the preexperiment, the postexperiment was conducted and 235 subjects participated.
Because of issues with the quality of the imaging data or a lack of effective data related to psychological variables, cross-sectional imaging analyses of the amount of time spent with parents were performed with 262 subjects (127 boys and 135 girls) and longitudinal imaging analyses were performed with 208 subjects (106 boys and 102 girls).
Although we have gathered the data of the Child Behavior Checklist, we have not excluded the subjects based on the score of this test in this project (Taki et al., 2010, 2012a, b; Takeuchi et al., 2013c), which is consistent with studies reported by some of other groups considered leading experts in the field (Kadosh et al., 2013; Sucksmith et al., 2013; Ullman et al., 2014). This is partly because this criterion would exclude many subjects who do not suffer from their problems so much that they do not feel the necessity to visit a hospital for their cure. Furthermore, for these measures, high continuity of nature between normality and abnormality has been consistently shown (O'Connor, 2002). Therefore, there are no practical reasons to exclude children on the basis of this score. Moreover, it has been suggested that the amount of parent–child interaction time facilitates the socioemotional development in children (NICHD Early Child Care Research Network, 2003). Thus, particularly for this study, excluding or correcting these effects may eliminate the effects due to the amount of parent–child interaction time and would therefore not be appropriate. Nevertheless, to check this procedure affects results and conclusions of this study, we analyzed the data without subjects with a T-score >70 on any subscale from the Child Behavior Checklist (Waber et al., 2007). Using this criterion yielded the exclusion of additional 18 subjects and the subsequent analysis demonstrated that the main significant results of this study (Table 1, analyses of 1, 6, 11, 12, and 19) are mostly not changed. Two results' statistical values slightly increased, whereas 3 results' statistical values slightly decreased. However, except for the effects of parent–child interaction on the Verbal Comprehension Index score in the cross-sectional analysis (where p value increased from 0.031 to 0.066), all the results remained significant (p < 0.05). These results did not alter our conclusions.
Table 1.
Statistical analyses performed in this study and the corresponding p value after correcting for study-wise multiple comparisonsa
| Question, dependent variable, independent variable | Type | p value* | |
|---|---|---|---|
| 1 | Does PCI (DV) associate with higher VCI (IV) as predicted from previous studies? | One-tailed | 0.031, 0.045 |
| 2 | Does PCI (DV) associate with POI (IV)? | Two-tailed | 0.891, 0.528 |
| 3 | Does PCI (DV) associate with PS (IV)? | Two-tailed | 0.825, 0.528 |
| 4 | Does PCI (DV) associate with WM (IV)? | Two-tailed | 0.276, 0.232 |
| 5 | Does PCI (DV) associate with FSIQ (IV)? | Two-tailed | 0.147, 0.138 |
| 6 | Does PCI (DV) associate with larger VCI increase (IV) as predicted from previous studies? | One-tailed | 0.020, 0.045 |
| 7 | Does PCI (DV) associate with POI change (IV)? | Two-tailed | 0.209, 0.186 |
| 8 | Does PCI (DV) associate with PS change (IV)? | Two-tailed | 0.881, 0.528 |
| 9 | Does PCI (DV) associate with WM change (IV)? | Two-tailed | 0.707, 0.514 |
| 10 | Does PCI (DV) associate with FSIQ change (IV)? | Two-tailed | 0.058, 0.058 |
| 11 | Does PCI (DV) associate with rGMD in the left STG? (IV)? | F-contrast | 0.015, 0.045 |
| 12 | Does PCI (DV) associate with rGMD in the right STG (IV)? | F-contrast | 0.038, 0.047 |
| 13 | Does PCI (DV) associate with rGMD in the left IFG (IV)? | F-contrast | 0.881, 0.528 |
| 14 | Does lower rGMD in the left STG (correlate of higher PCI) (IV) associate with advance development (DV)? | One-tailed | <1.63 × 10−11 **, 1.63 × 10−10 |
| 15 | Does lower rGMD in the right STG (correlate of higher PCI) (IV) associate with advance development (DV)? | One-tailed | 4.46 × 10−10, 3.57 × 10−9 |
| 16 | Does lower rGMD in the left STG (correlate of higher PCI) (IV) associate with superior VCI (DV) (another correlate of higher PCI)? | One-tailed | 0.038, 0.047 |
| 17 | Does lower rGMD in the right STG (correlate of higher PCI) (IV) associate with superior VCI (DV) (another correlate of higher PCI)? | One-tailed | 0.023, 0.045 |
| 18 | Does longitudinal rGMD change in the left STG (IV) associate with PCI (DV) like cross-sectional rGMD? | One-tailed | 0.963, 0.546 |
| 19 | Does longitudinal rGMD change in the right STG (IV) associate with PCI (DV) like cross-sectional rGMD? | One-tailed | 0.029, 0.045 |
| 20 | Does VCI (correlate of higher PCI) (IV) show the interaction effects between age and PCI (DV)? | — | 0.990, 0.546 |
| 21 | Does VCI change (correlate of higher PCI) (IV) show the interaction effects between age and PCI (DV)? | — | 0.799, 0.528 |
| 22 | Does mean rGMD of the PCI correlate in the left STG (IV) show the interaction effects between age and PCI (DV)? | — | 0.041, 0.047 |
| 23 | Does mean rGMD of the PCI correlate in the right STG (IV) show the interaction effects between age and PCI (DV)? | — | 0.023, 0.045 |
| 24 | Does mean rGMD change of the PCI correlate in the right STG (IV) show the interaction effects between age and PCI (DV)? | — | 0.667, 0.508 |
| 25 | Does VPCI (DV) correlate with VCI (IV) in a similar way as PCI? | One-tailed | 0.030, 0.045 |
| 26 | Does VPCI (DV) correlate with VCI change (IV) in a similar way as PCI? | One-tailed | 0.003, 0.016 |
| 27 | Does VPCI (DV) correlate with mean rGMD of the PCI's correlate in the left STG (IV) in a similar way as PCI? | One-tailed | 0.375, 0.300 |
| 28 | Does VPCI (DV) correlate with mean rGMD of the PCI's correlate in the right STG (IV) in a similar way as PCI? | One-tailed | 0.045, 0.047 |
| 29 | Does VPCI (DV) correlate with mean rGMD change of the PCI's correlate in the right STG (IV) in a similar way as PCI? | One-tailed | 0.020, 0.045 |
aPCI, Parent–child interaction (time spent with parents); DV, dependent variable; IV, independent variable; VCI, Verbal Comprehension Index; POI, Perceptual Organization Index; PS, Processing Speed; WM, Working Memory; LIFG, left inferior frontal gyrus; LSTG, left superior temporal gyrus; RSTG, right superior temporal gyrus; VPCI, verbal parent–child interaction (conversation factor score).
*The left p values show the p values that are uncorrected (non–voxel-based imaging analyses) or corrected within the analyses (voxel-based imaging analyses); the right p value shows the p values that are corrected in a study-wise manner through FDR.
**The actual p value is lower, but the SPM cannot display the value. In the study-wise FDR test, we used this value, but using the lower p value than this value will not affect the significance or insignificance of the study-wise FDR.
Assessments of psychological variables.
In both the preexperiment and postexperiments, we measured the Full-Scale intelligence quotient (FSIQ) using the Japanese version of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) for subjects aged 16 years or older or the Wechsler Intelligence Scale for Children-Third Edition (WISC-III) for subjects younger than 16 years (Azuma et al., 1998). The tests were administered by trained examiners (Fujita et al., 2006). We calculated the Verbal Comprehension subscore along with the FSIQ, Perceptual Organization subscore, Working Memory subscore, and Processing Speed subscore for each subject from their WAIS/WISC scores. In the preexperiment, data on the amount of time spent with parents (it was expressed as “time doing something together or dealing with parents”) during weekdays and holidays were collected using a questionnaire with multiple-choice questions. Responses were selected from the following 12 options: 1, none; 2, a little; 3, within 30 min; 4, ∼30 min; 5, ∼45 min; 6, ∼1 h; 7, ∼1 h 30 min; 8, ∼2 h; 9, ∼2 h 30 min; 10, ∼3 h; 11, ≥3 h; and 12, have no way of telling. These choices were then transformed into the number of hours spent with parents (option 1 = 0; option 2 = 0.167; option 3 = 0.333; option 4 = 0.5; option 5 = 0.75; option 6 = 1; option 7 = 1.5; option 8 = 2; option 9 = 2.5; option 10 = 3; option 11 = 3.5). The average number of hours spent with parents on weekdays and holidays was used in the statistical analyses described below. Data from subjects who chose option 12 were removed from analyses involving the time spent with parents. This measure has been widely used in studies of parent–child interactions in Japan, including governmental and international investigations (Management and Coordination Agency Headquarters About Juveniles, 1996; Ohyama, 2001; Okamoto, 2002). In this study, the correlation coefficient between the values from the first and second questions was 0.658, suggesting reliability for the average number of hours spent with parents. The amount of time spent with children has been shown to be positively associated with understanding children (Ohyama, 2001), as well as the frequency of parent–child behaviors assessed by other methods (see Investigation of the effects of verbal parent–child interaction), thus suggesting the validity of this measure. The general validity of this type of estimation method from recalling has been shown in a previous study (Robinson, 1985). Although a more tedious method, such as the experimentally organized diary method, is preferable for the accurate measurement of time, direct estimation method is found to be able to be used to draw valid inferences about correlates of time use and to obtain ordinal rankings of groups of individuals with regard to their time use in specific activities (Robinson, 1985).
In addition, data on each subject's relationship with his/her parents were collected using a self-report questionnaire with multiple choice questions. Responses were selected from the following five options: 1, going well; 2, not going so bad; 3, not going so well; 4, going bad; 5, have no way of telling. Data from subjects who chose option 5 were removed from analyses involving the time spent with parents, and the rest of the answers were used in the analyses as reported. We used only one question to measure the parent–child relationship. However, when the same question is used together with four other relevant questions in the scale for parent–child satisfaction, the scale's reliability was considered high with a Cronbach's α of 0.93 (Toguchi, 2009). This indicates that additional questions do not provide additional information. Therefore, to make the questionnaire as short as possible, we used only one question. This satisfaction scale comprising five questions shows a moderate correlation with the subfactors of bonding scales (Takagi and Toguchi, 2006), and its criterion-related validity has been confirmed.
We only assessed the time spent with parents at the preexperiment because this project is a prospective longitudinal cohort study. To indicate causality at certain levels with analyses in a prospective longitudinal cohort study, it is important that certain variables measured in the preexperiment period predict (or precede) subsequent changes in other variables. Otherwise, the analyses cannot suggest causality greater than that of cross-sectional studies.
As with other large projects that use MRI with children, we could not establish a common time for conducting the experiments that was accessible to all participants. In the MRI experiment, it was difficult to scan multiple subjects in confined seasons. For example, children can spend more time with their parents during holidays, although we distinguished between time spent with parents on holidays, or other days, and evaluated them separately. However, during long vacations, children have more holidays, and the effects of spending time with parents may be more pronounced either during or just after a long vacation. In Japan, there are usually no long vacations (>10 d) for adults. We believe that, as long as the time spent with parents was evaluated separately between holidays and other days, the differences in the time of year the scans were conducted are not likely a confounding variable in the present analyses. Nonetheless, this might reduce the sensitivity of the analyses and may be a common limitation of larger MRI projects involving daily habits. However, when time spent with parents on holidays (which is more likely to be common across ages) is used in the analysis instead of average number of hours spent with parents, the main significant results of this study (Table 1, analyses of 1, 6, 11, 12, 19) are mostly not changed. Two results' statistical values slightly increased, whereas three results' statistical values slightly decreased. However, except for the effects of parent–child interaction on the Verbal Comprehension Index score in the cross-sectional analysis (where p value increased from 0.031 to 0.101), all the results remained significant (p < 0.05). These results did not alter our conclusions.
In the preexperiment, the measure of socioeconomic status consisted of three questions. One was an inquiry relating to family annual income as reported in our previous study (Taki et al., 2010; Takeuchi et al., 2014). Annual income data were collected using discrete variables: 1, annual income <US $20,000 (the currency exchange rate was set at US $1 = 100 yen); 2, annual income US $20,000–$40,000; 3, annual income US $40,000–$60,000; 4, annual income US $60,000–$80,000; 5, annual income US $80,000–$100,000; 6, annual income US $100,000–$120,000; 7, annual income ≥US $120,000. The values 1–7 were used in subsequent regression analyses. The other two questions related to the highest educational qualification of both parents. There were eight options (1, elementary school graduate or below; 2, junior high school graduate; 3, normal high school graduate; 4, graduate of a short-term school completed after high school (such as a junior college); 5, university graduate; 6, masters degree; and 7, doctorate) and each choice was converted into the number of years taken to complete the qualification in the normal manner in the Japanese education system (1, 6 years; 2, 9 years; 3, 12 years; 4, 14 years; 5, 16 years; 6, 18 years; 7, 21 years). The average of the converted values for each parent was used in the analyses. This protocol followed the standard approach used by the Japanese government for evaluating socioeconomic status. Furthermore, according to previous work (Lederbogen et al., 2011), the urbanicity of the place (at the municipal level) where subjects lived when the first experiment was performed was scored in three categories: cities with >100,000 inhabitants (3), towns with >10,000 inhabitants, (2) and rural areas (1). The original publication (Pedersen and Mortensen, 2001) also included categories for (5) capital (4) and capital suburb, which did not occur in our sample. Further, none of the subjects in this study lived in rural areas. Even when another type of classification was used that was based on the thresholds of 1,000,000 or 100,000 inhabitants, the statistical values were less affected (data not shown). We also gathered data that were used to examine whether the mother and father lived together with the participants at the time of the first experiment and documented the number of parents living with participants. These data were included in the analyses as covariates.
For participants in the fourth grade or below, the parents (and not children) answered questions regarding the amount of parent–child interaction and the relationship between children and parents. For participants in the fifth grade or above, children themselves (and not parents) answered questions regarding his or her interactions with parents. This threshold is based on the low reliability of answers from small children, and the line was drawn between fourth and fifth grades according to the customs in the field, and previous recommendations were for discouraging the use of self-report methods due to their lack of validity and their use in children younger than 10 years (Kambara et al., 1998; Kohl et al., 2000). All other questions were answered by parents regardless of a child's grade.
Behavioral data analysis.
The behavioral data were analyzed using Predictive Analysis SoftWare release version 22.0.0 (PASW Statistics 18; SPSS 2010). Multiple regression analyses were used to investigate associations between hours spent with parents and cognitive functions in the preexperiment as well as associations between hours spent with parents and pretest to post-test changes in cognitive functions after correcting for the effects of confounding variables. Results with a threshold of p < 0.05 were considered statistically significant.
We did not include FSIQ as a covariate in the multiple regression analyses in the psychological analyses that examined whether spending time with parents affected the subscales of IQ tests. Including the IQ score is statistically incorrect, partly because of the multicolinearity problem (the r of the zero-order correlation between the FSIQ and Verbal Comprehension Index (VCI) score was as high as 0.83 in the present study) and because VCI is a part of the total IQ score, which are therefore fundamentally “overlapping.” In other words, conceptually the identical components exist between two variables. However, we cannot conceptually “regress out” the effects of IQ from VCI with multiple regression analyses. The same holds true for the longitudinal VCI and FSIQ changes (r = 0.737 in this case). Thus, we are unable to regress out the FSIQ change when we analyze the association between time spent with children and longitudinal VCI change (or changes of any other subscales). Nevertheless, we added the FSIQ score for the preexperiment in the longitudinal analyses of changes in subscales of the FSIQ because of the lack of the aforementioned problems and the concern that children with higher or lower cognitive functions may attract parents and increase the parent–child interaction. Such preexisting cognitive functions in children, rather than parent–child interactions, might cause developmental differences.
One-tailed tests were used in the VCI analyses because an association between parent–child interactions and development of verbal skills and knowledge was previously confirmed. The aim of these VCI analyses is to confirm the previously observed associations, and the analyses are hypothesis-driven. Two-tailed tests were used for other scores of the Wechsler IQ test because the analyses are not hypothesis-driven. Furthermore, we are unaware of previous studies that demonstrate an association between parent–child interactions and development of FSIQ, Working Memory, Perceptual Organization, or Processing Speed.
Image acquisition.
All images were collected using a 3-T Philips Intera Achieva scanner. Three-dimensional, high-resolution, T1-weighted images (T1WI) were collected using a MPRAGE sequence. The parameters were as follows: 240 × 240 matrix, TR = 6.5 ms, TE = 3 ms, TI = 711 ms, FOV = 24 cm, 162 slices, 1.0 mm slice thickness, and scan duration of 8 min and 3 s.
Preprocessing and analysis of structural data.
Preprocessing of the structural data was performed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, London) implemented in MATLAB (MathWorks). Using the new segmentation algorithm implemented in SPM8, T1-weighted structural images of each individual were segmented into six tissues. In this process, the gray matter tissue probability map (TPM) used in this procedure was manipulated from maps implemented in the software so that the signal intensities of the voxels with (gray matter tissue probability of the default tissue gray matter TPM + white matter tissue probability of the default TPM) <0.25 became 0. When this manipulated gray matter TPM is used, the dura matter is less likely to be classified as gray matter (compared with when the default gray matter TPM is used) without other substantial segmentation problems. In this new segmentation process, default parameters were used, except that affine regularization was performed using the International Consortium for Brain Mapping template for East Asian brains. We then proceeded into the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) registration process implemented in SPM8. In this process, we used DARTEL-imported images of the 6 TPM of gray matter created using the abovementioned new segmentation process. First, the template for the DARTEL procedures was created using the T1WI data from the prescan of all the subjects. Next, using this existing template, DARTEL procedures were performed using the prescan and postscan T1WI of all of the subjects included in this study and default parameter settings. The resulting images were then spatially normalized to the MNI space to obtain images with 1.5 × 1.5 × 1.5 mm3 voxels. Subsequently, all images were smoothed by convolving them with an isotropic Gaussian kernel of 12 mm FWHM for the reasons described below.
Finally, the signal change in rGMD between preintervention and postintervention images was computed at each voxel for each participant. In this computation, the preexperiment's unsmoothed rGMD images were subtracted from the postexperiment's unsmoothed rGMD images, and the resulting images were then smoothed by convolving them with an isotropic Gaussian kernel of 12 mm FWHM. The resulting maps representing the rGMD between the pre-MRI and post-MRI experiments (rGMD post − rGMD pre) were then forwarded to the longitudinal imaging analyses, as described in the following section.
Statistical analysis of imaging data.
Statistical analyses of imaging data were performed with VBM5 software, an extension of SPM5, for the reasons described below.
In the cross-sectional analyses of rGMD, we included only voxels that showed rGMD values of >0.10 in all subjects. In longitudinal analyses, we only included voxels that had rGMD values >0.10 in both the presmoothed and postsmoothed rGMD images for all subjects. The primary purpose for using this type of threshold is to cut the periphery of the gray matter area and to effectively limit the area for analysis to areas likely to be gray matter.
Cross-sectional whole-brain multiple regression analysis was performed to investigate the association between rGMD and the time spent with parents. Sex, age (days after birth), family annual income, average number of years of parents' highest educational qualification, urbanicity of the areas in which participants lived, number of parents who lived together with the participants, relationship with parents, whether the parent or participant answered the questions regarding the interaction between parents and children, and number of hours spent with parents (as calculated above) were independent variables. Thus, there were nine independent variables in total, and rGMD at each voxel was the dependent variable. The centering option was used to center the interactions.
Next, to reveal the nature of the rGMD correlates of hours spent with parents in terms of the association with verbal abilities and development, we investigated the association between rGMD and the Verbal Comprehension score as well as the association between rGMD and age using voxel-by-voxel multiple regression analysis. In this multiple regression analysis, sex, age (days after birth), and Verbal Comprehension were independent variables and rGMD at each voxel was the dependent variable. The FSIQ or VCI was not included in this model because they are concurrent phenomena with rGMD. Furthermore, rGMD correlates with these cognitive functions are included among what we would like to investigate in the analyses of associations between parent–child interactions and rGMD.
In longitudinal analyses of rGMD, the maps representing signal changes in rGMD between the preexperiment and postexperiment images were analyzed. We investigated the association between preexperiment and postexperiment rGMD changes and hours of time spent with parents after regressing out age, length of time between the preexperiment and postexperiment, sex, family annual income, average number of years of parents' highest educational qualification, the urbanicity of the areas in which participants lived, number of parents who lived together with participants, FSIQ in the preexperiment, whether the parent or participant answered the questions regarding the interactions between parents and children, relationship with parents, number of hours spent with parents, and rGMD at each voxel in the preexperiment using the biological parametric mapping toolbox for SPM (Casanova et al., 2007). The BPM toolbox can handle voxel-by-voxel correlations between multimodal images, and it was used to correct the effect of rGMD in the preexperiment on the preexperiment to postexperiment rGMD changes at each voxel. Changes in FSIQ or VCI from the preexperiment to postexperiment measures were not included in the model of longitudinal rGMD analyses for the same reasons that the cross-sectional rGMD analyses of FSIQ or VCI score in the preexperiment are described above.
In the cross-sectional whole-brain analyses, the statistical significance level was set at p < 0.05, corrected at the nonisotropic adjusted cluster level (Hayasaka et al., 2004) with an underlying voxel level of p < 0.0025. Nonisotropic adjusted cluster-size tests can and should be applied when cluster size tests are applied to data known to be nonstationary (in another words, not uniformly smooth), such as VBM data (Hayasaka et al., 2004). In this nonisotropic cluster-size test of random field theory, a relatively higher cluster-determining threshold combined with high smoothing values of >6 voxels leads to appropriate conservativeness in real data. With high smoothing values, an uncorrected threshold of p < 0.01 seems to lead to anticonservativeness, whereas that of p < 0.001 seems to lead to slight conservativeness (Silver et al., 2011); thus, the abovementioned threshold was used. We used the VBM5/SPM5 version of this test and a smoothing value of 12 mm. This is because a previous validation study of this test using a real dataset (Silver et al., 2011) showed that the conditions of this cluster size test are very limited and are dependent on the smoothness of the data as described above. However, SPM8 and SPM5 estimate actual FWHM substantially differently in the areas analyzed and this directly affects the cluster test threshold. Therefore, regardless of which (SPM5 or SPM8) is appropriate, our view is that the conditions shown in the previous study (Silver et al., 2011) are no longer guaranteed in SPM8 because different analyses are performed and these return different results. We used a 12 mm FWHM, which was recommended in the previous study (Silver et al., 2011). In addition, the use of a 12 mm FWHM for rGMD images resulting from DARTEL procedures under the current conditions appears to be sufficient for achieving actual smoothness (i.e., the amount of smoothness in analyses of the acquired data when the recommended smoothing value is used with segmented VBM images from previous versions). In longitudinal analyses, because of incompatibility between SPM and the nonisotropic adjusted cluster size test, nonisotropic adjusted cluster size tests could not be performed; therefore, we focused on ROI analyses. However, whole-brain longitudinal analyses did not result in any significant findings, regardless of the method used for correcting for multiple comparisons across the whole brain.
In the cross-sectional and longitudinal voxel-by-voxel multiple regression analyses, for areas with a strong a priori hypothesis, namely, the IFG, and STG the statistical significance level was set at p < 0.05, with a small-volume correction (SVC) for multiple comparisons (family-wise error) in regions of interest. Detailed reasons for these regions being chosen were described in the Introduction. All ROIs were constructed using the WFU PickAtlas Tool (http://www.fmri.wfubmc.edu/cms/software#PickAtlas) (Maldjian et al., 2003, 2004) and was based on the PickAtlas automated anatomical labeling atlas option (Tzourio-Mazoyer et al., 2002). The masks of the bilateral IFG were constructed by adding mask images from subregions of IFG, and the masks of the bilateral STG were constructed according to automated anatomical labeling.
Interaction effects between age (who answered the question on parent–child interaction) and number of hours spent with parents and their impact on significant correlates of time spent with parents.
To investigate whether psychological and anatomical correlates of the time spent with parents were driven by a particular age group (in other words, to test whether the associations between the time spent with parents and its correlates differ between younger and older children), we performed additional analyses using ANCOVAs in PASW Statistics 22. The dependent variables in both analyses were the significant correlates of hours spent with parents identified in the present study (i.e., one of the following: Verbal Comprehension in cross-sectional and longitudinal analyses and mean rGMD value for significant clusters identified in cross-sectional and/or longitudinal analyses). In these analyses, the subjects were divided into two groups on the basis of whether they were ≤fourth grade or ≥fifth grade, which corresponds to who answered the question on parent–child interactions (guardians or children themselves). We added the interaction between the number of hours spent with parents and this group factor as covariates in the models for each analysis in addition to all other covariates that were used in each of abovementioned analysis of correlates of hours of spending time with parents.
We did not have an a priori hypothesis for these analyses. Thus, we simply applied the p values of interaction effects from the ANCOVAs through F-tests.
Investigation of the effects of verbal parent–child interactions.
As was the case with our previous studies, we assessed the time spent with parents but did not confine such time to verbal interactions. The present effects of time spent with children on verbal skills and verbal-related neural systems might be caused by verbal parent–child interactions. To confirm such notions, we supplementarily performed the following analyses using ANCOVAs in PASW Statistics 22.
In the present study, we used the data obtained with a set of questions that examined the frequencies of various parent–child interactions. For each item, the content (e.g., “have dishes”) was shown, and the participant (or parent) was asked how often the parents and children together behaved in such a manner. Responses were selected from the following four options: 1, not at all; 2, relatively infrequent; 3, relatively frequent; 4, frequent. These answers were used as reported. The questions have been used in studies of parent–child interactions in Japan, including governmental and international investigations, although the exact contents of the items vary among studies (Management and Coordination Agency Headquarters About Juveniles, 1996; Ohyama, 2001; Okamoto, 2002).
Because there are 16 items, we first performed a promax-rotated factor analysis (unweighted least-squares method) of scores for each question in this set of questions. Based on the recommendation of Stevens (1996), the Scree test was used to determine the number of factors. As a result, four-factor solution was selected. Eigenvalues of the first six factors were 4.33, 2.13, 1.5, 1.12, 0.84, and 0.83. The recommended cutoff value of factor loadings to include certain items in the factor recommended is sometimes >0.4 (Ishii, 2005). Therefore, we used this cutoff value for factor loadings.
The first factor comprised six items that we named the “small children” factor. This factor consisted of acts that parents usually do only with small children and includes items, such as “take care of clothes” (factor loading, 0.479), “take a bath” (0.674), “sleep together at night” (0.573), “parents read books to children”(0.750), “play pretend games using toys and tools” (0.722), and “parents teach children” (0.597). The second factor consisted of three items that we named the “conversation” factor. This factor consisted of items, such as “talk about what happened today” (0.603), “talk about future path” (0.840), and “talk about life” (0.752). The third factor consisted of three items that we named the “play together” factor. This factor consisted of items such as “play games indoors” (0.420), “go for a walk or play in the park” (0.755), and “play sports” (0.744). The fourth factor consisted of two items and we named the “have dishes and watch TV or listen to music” factor. This factor included items, such as “have dishes” (0.533) and “watch TV or listen to music” (0.527). The items “go shopping” and “make dishes” were removed based on the criteria of factor loading and the other 14 items belonged to one of the abovementioned factors.
Apparently, the “conversation” factors matched with our interest. The correlation coefficients (r) between time spent with parents and the factors were 0.296 (“small children”), 0.390 (“conversation”), 0.201 (“play together”), and 0.210 (“have dishes and watch TV or listen music”). These r values demonstrate the cross-validity of the four factors and the amount of time spent with children; the “conversation” factor showed the strongest association with the amount of time spent with parents. The Cronbach's α value was 0.772 for the “small children” factor, 0.764 for the “conversation” factor, 0.645 for the “play together” factor, and 0.396 for the “have dishes and watch TV or listen music” factor. Therefore, the first three factors have good to acceptable reliability, but the fourth factor may have a problem with reliability. However, our focus is the “conversation” factor, which represents verbal parent–child interactions, and the reliability of the fourth factor is less of an issue.
We then proceeded to multiple regression analyses using the “conversation” factor as a measure of verbal parent–child interaction. The dependent variables in both psychological and anatomical analyses were the significant correlates for hours spent with parents (i.e., one of the following: Verbal Comprehension in cross-sectional and longitudinal analyses and mean rGMD value for significant clusters identified in cross-sectional and longitudinal analyses). We added the “conversation” factor as a covariate in the models for each analysis, and in addition to all other covariates that were used in the correlational analyses of hours of time spent with parents, and instead removed time spent with parents from the model.
We used one-tailed tests in the present analyses because their purpose was to confirm that verbal parent–child interaction has the same association patterns with correlates of parent–child interaction (time spent with children), rather than confirm the verbal parent–child interaction has any association with correlates of parent–child interaction.
Study-wise multiple comparison issues.
Although this study has only a few analyses that directly addressed the hypotheses (cross-sectional and longitudinal imaging analyses involving time spent with children), there are numerous other analyses. Some of them support the discussion and interpretation of the present results. Other analyses were added to show specificity of the main findings or the readers' interest. Therefore, there may be concerns with multiple comparisons issues.
To our knowledge, there are no established methods to correct multiple comparisons across multiple voxel based and non–voxel-based analyses. Thus, the method for such an analysis is somewhat exploratory. Basically, we used the false discovery rate (FDR), as was the case in a prior imaging study (Wallace et al., 2010), although that study did not deal with voxel-based analyses. We confirmed whether the present results were within a threshold of p < 0.05, and corrected for FDR using the graphically sharpened method (Benjamini and Hochberg, 2000) when multiple comparisons were performed in a study-wise manner. FDR-based methods have been shown to be more powerful and sensitive than other available approaches to multiple statistical testing (for a full discussion, see Benjamini and Hochberg, 1995; Genovese et al., 2002).
In this calculation, for non–voxel-based imaging analyses, the p values of that were unchanged from abovementioned analyses used for the computation of p values that are corrected for FDR. However, in traditional voxel-based imaging analyses and abovementioned voxel-based imaging analyses in this study, the results are reported with one-tailed tests, in this calculation of study-wise FDR-based p values, we did so only in cases where there were hypotheses regarding the directionality of the associations (in cases where analyses were performed to confirm associations in a specific direction, rather than confirming there are associations in any direction). In other voxel-based imaging analyses (i.e., analyses that did not have any hypotheses or did not test the association of the certain directions), in this calculation of study-wise FDR-based p values, we tested the associations in F-contrasts (and voxelwise correction for multiple comparisons using family wise error) for each ROI. Because this analysis becomes complicated when there are multiple significant results in one-voxel based imaging analysis, in this calculation of study-wise FDR-based p values, we just picked up only the strongest result or voxel in each analysis for each area.
In the imaging analyses described in Statistical analysis of imaging data, the cross-sectional associations between time spent with parents and rGMD in the three ROIs were two-tailed. We selected two-tailed analyses because some studies have associated children's adaptive characteristics with less gray matter in the ROIs in terms of associations between parents and children (De Bellis et al., 2002a; Tomoda et al., 2011). However, this was not always the case (De Brito et al., 2009), and that may depend on the samples' characteristics.
The subsequent analyses of associations between age and rGMD, as well as between VCI and rGMD, were performed to confirm that age and VCI have the same association patterns with rGMD in the bilateral temporal gyrus, which was associated with time spent with children (and not to investigate age and VCI have any associations with rGMD in these areas). The same holds true to longitudinal analyses between rGMD change and time spent with children.
Results
Basic data
The characteristics of the subjects are shown in Table 2. The amount of time spent with parents was determined using a self-report questionnaire. We used ANOVA to investigate whether the psychological variables measured in the preexperiment that were used in the multiple regression analyses were different between subjects who only participated in the preexperiment and those who participated in both the preexperiment and postexperiment. There were no significant differences in these variables (p > 0.05, uncorrected).
Table 2.
Psychological variables of the study participants in the cross-sectional analyses (127 boys and 135 girls, upper lines) and their change in the longitudinal analyses (106 boys and 102 girls, lower lines when there are two lines)
| Measure | Boys | Girls |
|---|---|---|
| Age (yr) | 10.8 ± 2.9, 5.7–16.5 | 11.4 ± 3.4, 5.8–18.4 |
| 3.0 ± 0.3, 1.7–4.0 | 3.0 ± 0.3, 1.8–4.1 | |
| FSIQ | 104.4 ± 12.9, 77–134 | 102.0 ± 11.3, 71–128 |
| 0.4 ± 8.6, −18 to 23 | 1.9 ± 9.5, −44 to 26 | |
| Verbal Comprehension | 104.9 ± 13.9, 64–145 | 103.1 ± 14.0, 65–136 |
| 0.4 ± 8.6, −36 to 27 | 2.0 ± 11.2, −36 to 29 | |
| Working Memory | 102.5 ± 13.1, 71–138 | 98.6 ± 12.4, 69–124 |
| −0.7 ± 9.3, −18 to 23 | −1.4 ± 11.5, −61 to 18 | |
| Perceptual Organization | 102.9 ± 15.3, 69–146 | 100.0 ± 11.9, 68–134 |
| 0.0 ± 10.5, −29 to 23 | 0.2 ± 11.5, −48 to 31 | |
| Processing Speed | 100.4 ± 11.8, 66–134 | 104.4 ± 13.2, 69–136 |
| 5.7 ± 10.6, −28 to 34 | 5.4 ± 12.6, −30 to 39 | |
| Family annual incomea | 4.09 ± 1.51, 1–7 | 3.88 ± 1.48, 1–7 |
| Average number of years of parents' highest educational qualification | 14.3 ± 1.74, 9–18.5 | 14.1 ± 1.55, 10.5–18.5 |
| Average hours of spending time with parents for weekdays and holidays (hours) | 2.24 ± 1.01, 0–3.5 | 2.34 ± 1.02, 0.08–3.5 |
aData are mean ± SD, range. Family annual income was classified as follows: 1, annual income <2 million yen; 2, 2–4 million yen; 3, 4–6 million yen; 4, 6–8 million yen; 5, 8–10 million yen; 6, 10–12 million yen; 7, >12 million yen.
Cross-sectional behavioral analysis
A multiple regression analysis that used preexperiment data revealed (after correcting for the effects of age, sex, family annual income, the average number of years of parents' highest educational qualification, the urbanicity of the areas in which the participants lived, number of parents who lived together with the participants, and whether the participant or parents answered the questions regarding the interaction between parents and children, and the relationship with parents) that the number of hours spent with parents in the preexperiment showed a significant and positive correlation with the Verbal Comprehension scores of WAIS-III and WISC-III in the preexperiment (p = 0.031, t = 1.876, standardized partial regression coefficient [β] = 0.120), as expected. However, the association between Verbal Comprehension and the number of hours spent with parents did not show a correlation with the Perceptual Organization (p = 0.891, t = 0.137, β = 0.009), Processing Speed (p = 0.825, t = 0.222, β = 0.014), Working Memory (p = 0.276, t = 1.092, β = 0.067), or FSIQ (p = 0.147, t = 1.454, β = 0.093) scores of WAIS-III and WISC-III in the preexperiment.
Longitudinal behavioral analysis
A multiple regression analysis performed using the longitudinal data revealed (after correcting for the effects of age, sex, family annual income, average number of years of parents' highest educational qualification in the preexperiment, the urbanicity of the areas in which the participants lived, number of parents who lived together with the participants, whether the participant or parents answered the questions regarding the interaction between parents and children, relationship with parents, duration of time between the postexperiment and preexperiment, and score for each test in the preexperiment) that the number of hours spent with parents in the preexperiment was significantly and positively correlated with the change in the Verbal Comprehension subscore between the preexperiment and postexperiment data (p = 0.020, t = 2.075, β = 0.109). However, this association was again specific to the Verbal Comprehension score and was not significantly correlated with the change in the Perceptual Organization (p = 0.209, t = 1.261, β = 0.088), Working Memory (p = 0.881, t = 0.150, β = 0.008), Processing Speed score (p = 0.707, t = 0.377, β = 0.022), or FSIQ (p = 0.058, t = 1.903, β = 0.093) between the preexperiment and postexperiment data.
Cross-sectional rGMD analysis
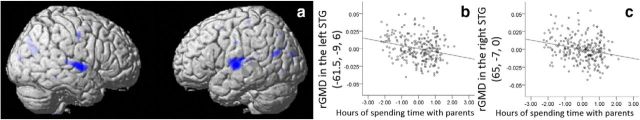
A multiple regression analysis that used preexperiment data revealed (after correcting for the effects of age, sex, family annual income, the average number of years of parents' highest educational qualification, the urbanicity of the areas in which the participants lived, number of parents who lived together with participants, whether the participant or parents answered the questions regarding the interaction between parents and children and the relationship with parents) that the number of hours spent with parents in the preexperiment was significantly and negatively correlated with rGMD in an anatomical cluster that spread from the right STG and Heschl's gyrus (MNI coordinates x, y, z = 65, −7.5, 0, t value of the peak = 3.99, p = 0.010, corrected for multiple comparisons at the nonisotropic adjusted cluster level; Fig. 1a–c) and in an anatomical cluster that spread from the left STG, across the middle temporal gyrus, to Heschl's gyrus (MNI coordinates x, y, z = −61.5, −9, 6, t value of the peak = 4.18, p = 0.026, corrected for multiple comparisons at the nonisotropic adjusted cluster level; Fig. 1a–c). There were no other significant results in the whole-brain analysis of rGMD. Small-volume corrections were used among areas with a strong a priori hypothesis. However, this analysis failed to show significant correlations in the whole-brain analysis (i.e., left IFG), and there were no significant results in this analysis. Although we did not include FSIQ as a covariate in this analysis (for the reasons, see Materials and Methods), the anatomical cluster around the right STG remained significant at the whole-brain level, even when FSIQ was added as a covariate. In this additional analysis that used FSIQ as a covariate, the anatomical cluster in the left STG became just a tendency (p < 0.1, corrected) at the whole-brain level; and when the SVC was applied, there were still significant results in the left STG.
Figure 1.
Negative rGMD correlates of the amount of time (hours) spent with parents in cross-sectional analyses. a, Negative rGMD correlates of time spent with parents. Significant correlations in the left temporal area and the right STG were observed together with other nonsignificant correlations. Results are shown with p < 0.0025, uncorrected for visualization purposes. b, c, Associations between rGMD and the amount of time spent with parents. Residual plots with trend lines depicting correlations between residuals in multiple regression analyses with rGMD of the peak voxels in (b) the right STG and (c) the left STG as the dependent variables.
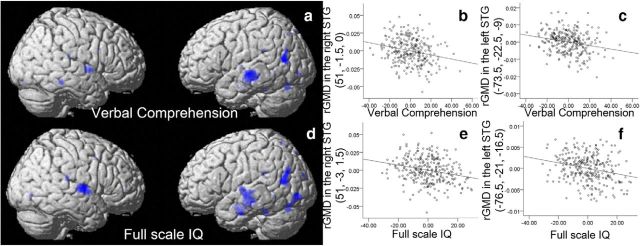
To reveal the nature of the rGMD correlates of Verbal Comprehension, which was significantly associated with the number of hours spent with parents, we used multiple regression analysis for investigating the association between rGMD and Verbal Comprehension scores. A multiple regression analysis that used preexperiment data revealed (after correcting for the effects of age and sex) that the Verbal Comprehension score showed significant negative correlation with rGMD in an anatomical cluster that spread through the left superior and middle temporal gyrus (MNI coordinates x, y, z = −74, −22, −9, t value of the peak = 4.31, p = 0.038, corrected for multiple comparisons at the nonisotropic adjusted cluster level; Fig. 2a,b,d). Small-volume corrections were used among areas with a strong a priori hypothesis, and a significant negative correlation was observed between the Verbal Comprehension score in the preexperiment and rGMD of the right STG (MNI coordinates x, y, z = 51, −1, 0, t value of the peak = 3.92, p = 0.023, corrected for FWE at the voxel level within the areas with a strong a priori hypothesis; Fig. 2a–c). The clusters in the bilateral temporal gyrus in which rGMD was significantly correlated with the number of hours spent with parents did not overlap with the clusters in which rGMD was significantly correlated with the Verbal Comprehension score at the applied threshold; however, they overlapped when the clusters were formed at a threshold of p < 0.01, uncorrected. Also, the additional referencial analysis replacing Verbal Comprehension score with FSIQ revealed the similar negative correlation pattern in and around areas of the bilateral superior temporal gyrus (Fig. 2).
Figure 2.
Negative rGMD correlates of Verbal Comprehension and FSIQ in cross-sectional analyses. a, Negative rGMD correlates of Verbal Comprehension. Significant correlations were observed in the left temporal area and the right STG together with other nonsignificant correlations. Results are shown with p < 0.0025, uncorrected for visualization purposes. b, c, Associations between rGMD and Verbal Comprehension. Residual plots with trend lines depicting the correlations between residuals in multiple regression analyses with rGMD of the peak voxels in (b) the right STG and (c) the left STG as the dependent variables. d, Negative rGMD correlates of FSIQ. Significant correlations were observed in the left temporal area and the right STG together with other nonsignificant correlations. Results are shown with p < 0.0025, uncorrected for visualization purposes. e, f, Associations between rGMD and FSIQ. Residual plots with trend lines depicting the correlations between residuals in multiple regression analyses with rGMD of the peak voxels in (e) the right STG and (f) the left STG as the dependent variables.
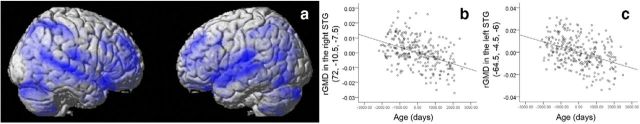
In addition, to reveal the nature of the association between rGMD of STG and development, we investigated rGMD correlates of age in the same multiple regression analysis. After correcting for the effects of sex and Verbal Comprehension score, age was found to be significantly and negatively correlated with a wide range of areas in the whole brain (p < 0.05, corrected for multiple comparisons at the nonisotropic adjusted cluster level; Fig. 3a,b). In this analysis, the cluster became too large and the cluster-based test became inappropriate because of the nature of the cluster-based test (it does not indicate exactly where the effect of interest is located within a cluster). The extent of the clusters in which rGMD was negatively correlated with age substantially overlapped the clusters in which rGMD was negatively correlated with the amount of time spent with parents described above (Fig. 3a–d).
Figure 3.
Negative rGMD correlates of age in cross-sectional analyses. a, b, Negative rGMD correlates of age. Correlations in the bilateral STG were observed together with correlations in other areas. a, Results are shown with p < 0.0025, uncorrected for visualization purposes. b, c, Associations between rGMD and age. Residual plots with trend lines depicting the correlations between residuals in the multiple regression analyses with rGMD of the peak voxels in (b) the right STG and (c) the left STG as the dependent variables.
Longitudinal rGMD analysis
Next, we investigated the association between rGMD changes in the preexperiment to postexperiment analysis and the number of hours spent with parents in the preexperiment. Multiple regression analysis was performed with age, time between the preexperiment and postexperiments, sex, family annual income, average number of years of parents' highest educational qualification, the urbanicity of the areas in which participants lived, number of parents who lived together with participants, whether the participants or parents answered the questions regarding the interaction between parents and children, FSIQ in the preexperiment, relationship with parents, number of hours spent with parents in the preexperiment, and rGMD at each voxel in the preexperiment as independent variables and the preexperiment to postexperiment rGMD change as the dependent variable.
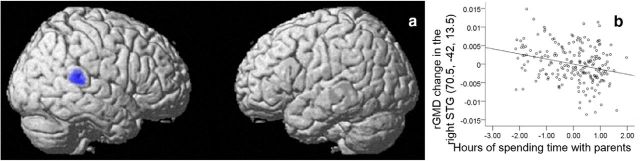
SVC were used among areas with a strong a priori hypothesis, and the number of hours spent with parents in the preexperiment was significantly and negatively correlated with the preexperiment to postexperiment change in rGMD of the right STG (MNI coordinates x, y, z = 70.5, −42, 13.5, t value of the peak = 3.85, p = 0.029, corrected for FWE at the voxel level within the areas with a strong a priori hypothesis; Fig. 4a,b). There were no other significant relationships. In this analysis, however, changes in FSIQ from preexperiment to postexperiment periods were not included as a covariate (for the reasons described in Materials and Methods). Even after including changes in FSIQ as a covariate, there was a significant result in the right STG in this SVC analysis.
Figure 4.
Negative rGMD correlates of the amount of time spent with parents in longitudinal analyses. a, Negative rGMD correlates of the amount of time spent with parents. Significant correlations were observed in the right STG. Results are shown with p < 0.0025, uncorrected for visualization purposes. b, Associations between rGMD and the amount of time spent with parents. Residual plot with trend lines depicting correlations between residuals in multiple regression analyses with rGMD of the peak voxels in the right STG as the dependent variables.
However, the cluster of the right STG in which a significant correlation was observed between the longitudinal rGMD change and the number of hours spent with parents did not overlap with the cluster in which a significant correlation was observed between rGMD of the right STG and the number of hours spent with parents or the cluster of the left STG in which a significant correlation was observed between rGMD and Verbal Comprehension. When a threshold of 0.01 uncorrected was used, a cluster of the right STG in which a negative correlation was observed between longitudinal rGMD change and the number of hours spent with parents substantially overlapped clusters in which correlations were observed between rGMD and the number of hours spent with parents in the preexperiment and between rGMD and the Verbal Comprehension score in the preexperiment (however, the cluster of the right STG in which the longitudinal correlation was observed was not contiguous with the significant cluster described above). On the other hand, the cluster of the right STG in which a significant correlation was observed between longitudinal rGMD change and the number of hours spent with parents substantially overlapped with the cluster in which a significant cross-sectional correlation was observed between rGMD and age, as described above.
Interaction effects of age (who answered the questions on parent–child interaction) and number of hours spent with parents on significant correlates of time spent with parents
We also investigated the interaction effects of age (who answered the question on parent–child interaction) and the number of hours spent with parents to determine their impact on the variables that showed significance or a tendency for association with time spent with parents in both cross-sectional and longitudinal analyses.
In the analyses of the psychological and longitudinal imaging correlates of time spent with parents, no significant interaction effects were observed between age and the number of hours spent with parents on psychological and imaging correlates of the spending time with parents (cross-sectional analysis of VCI; p = 0.990, F = < 0.001, longitudinal analysis of VCI; p = 0.799, F = 0.065, longitudinal analyses of the mean rGMD for the right STG of the significant cluster of longitudinal imaging correlates for spending time with parents; p = 0.667, F = 0.185).
In the analyses of the cross-sectional imaging correlates of the time spent with parents, we observed significant interaction effects of age (who answered the question on parent–child interaction) and the number of hours spent with parents on the mean rGMD of the left STG of the significant cluster of cross-sectional imaging correlates of the spending time with parents (p = 0.041, F = 4.201) and that of right STG (p = 0.023, F = 5.254). In both cases, the effects of spending time with parents were stronger in the younger age group. The simple correlation coefficients (r) between the mean rGMD value in the significant clusters and the number of hours spent with parents were as follows: mean rGMD in the significant cluster of the above-identified cross-sectional left STG in the younger group, r = −0.309; mean rGMD in the above-identified significant cross-sectional cluster of the left STG in the older group, r = −0.156; mean rGMD in the above-identified cross-sectional significant cluster of the right STG in the younger group, r = −0.322; mean rGMD in the above-identified cross-sectional significant cluster of the right STG in the older group, r = −0.107. When the interaction effects of age (who answered the question on parent–child interaction) and the number of hours spent with parents were replaced by the interaction effects of continuous age variable and number of hours spent with parents, there were no significant effects and the aforementioned significant interaction effects of age and the number of hours spent with parents on rGMD in the bilateral STG in the cross-sectional analyses became nonsignificant, although the tendencies remained (p < 0.1 for both of analyses).
However, the implication of these findings of significant interaction effects with age (who answered the question on parent–child interaction) is unclear. This is because: (1) these interaction effects were not clear in the longitudinal analyses; (2) we did not perform experiments to analyze these types of interaction effects, and the age of the subjects was continuous and not suited to this type of analysis; and (3) in this analysis, when the age was lower (under fourth grade), two variables changed at once (age and who answered the questions regarding parent–child interaction), thus, what causes (e.g., higher effects when children are smaller, or higher reliability of the parental report). Future studies are required to investigate this issue using a more suitable experimental design.
Investigation of the effects of verbal parent–child interaction on correlates of significant correlates of time spent with parents
We also investigated the effects of verbal parent–child interaction on the significant correlates of time spent with parents. These analyses were performed to test whether the present significant correlates of time spent with parents are caused by verbal parent–child interaction.
In the analyses of the cross-sectional psychological correlates of the time spent with parents (VCI score), we observed significant positive correlation between “conversation” factor scores of parent–child interaction and VCI scores (p = 0.030, t = 1.896). In the analyses of the longitudinal correlates of time spent with parents (i.e., changes of VCI score), we observed a significant positive correlation between “conversation” factor scores of parent–child interaction and changes in VCI score (p = 0.003, t = 2.779).
In the analyses of the cross-sectional neuroimaging correlates for the time spent with parents (i.e., mean rGMD of the left STG of the significant cluster of cross-sectional imaging correlates for spending time with parents and that of the right STG), we observed a significant negative correlation between “conversation” factor scores of parent–child interaction and mean rGMD of the significant cluster in the right STG (p = 0.045, t = −1.705). However, there was no significant association between the mean rGMD and the significant cluster in the left STG (p = 0.375, t = −0.319).
In the analyses of the longitudinal neuroimaging correlates of time spent with parents (i.e., mean rGMD of the right STG of the significant cluster of longitudinal imaging correlates of spending time with parents), we observed a significant negative correlation between “conversation” factor scores of parent–child interaction and the mean rGMD of the significant cluster in the right STG (p = 0.020, t = −2.048).
Study-wise multiple comparison issues
The results of the present analyses are presented in Table 1. Results that were significant in each analysis remained significant after accounting for the study-wise multiple comparisons using FDR. This was because most of the analyses in this study were not exploratory, and the majority of the analyses showed significant results in each contrast and nature of the FDR testing. The study-wise correction for multiple comparisons, including both voxel-based analyses and non–voxel-based analyses, is an exploratory method. However, when the majority of the analyses show significance in each analysis, corrections for multiple comparisons are of less concern. The conclusion that corrections for study-wise multiple comparisons are of less concern will not change.
Discussion
In the present study, we revealed for the first time the effects of the amount of parent–child interaction on children's brain structures using both cross-sectional and longitudinal analyses. Our hypothesis was partly confirmed and our cross-sectional studies newly revealed that the amount of time spent with parents was negatively correlated with rGMD in anatomical clusters involving the bilateral STG as well as an increase in rGMD in an area adjacent to the right STG a few years later. Congruent with the abovementioned previous studies, the amount of time spent with parents was found to be positively correlated with Verbal Comprehension in the cross-sectional studies, and a further increase in Verbal Comprehension was predicted a few years later. Verbal Comprehension was found to be negatively correlated with rGMD in similar contingent areas of the bilateral STG using cross-sectional analyses. Further, the amount of verbal parent–child interaction had similar effects on Verbal Comprehension and rGMD of the right STG in cross-sectional and longitudinal analyses. Based on these findings, the structure of the right STG may partly mediate the link between the amount of parent–child interaction and the development of Verbal Comprehension. These types of relationships were not observed in IFG, at least at the applied threshold, and no associations between rGMD and the amount of time spent with parents were observed in IFG in the present study. However, these clusters did not completely overlap at the stringent thresholds used, and the possible associations between cognitive and neural development brought about by parent–child interactions need to be interpreted with caution.
We found that spending time with children affects rGMD of the right STG and that this effect may be associated with the negative effect of spending time with parents on verbal ability. The amount of time spent with parents was negatively correlated with rGMD in the bilateral temporal gyrus and positively correlated with Verbal Comprehension in cross-sectional analyses. Furthermore, a larger amount of time spent with parents was associated with a reduction in rGMD of the right STG a few years later, although this was in a slightly different location and was positively correlated with developmental changes in Verbal Comprehension. On the other hand, rGMD of similar areas was negatively correlated with Verbal Comprehension. rGMD of this area also decreased as the children grew older. These results indicate that increased parent–child interactions affect this area and that any resultant changes may underlie or be associated with the effects of increased parent–child interactions on verbal skills. However, there was only a marginal overlap, if any, between the significant clusters for each effect at the applied threshold. These significant clusters were observed in similar areas; and as the statistical thresholds were made more lenient, the clusters overlapped at some stages. Thus, the weak overlap between significant clusters and the slight differences in locations may simply reflect the fact that the study had insufficient statistical power to detect associations between cognition and structures (Takeuchi et al., 2013a, b) and could also reflect the effects of daily habits in epidemiological studies. We cannot be sure about this, and our findings need to be interpreted with caution until they can be replicated.
The right STG is involved in a wide range of nonliteral information processing in communicative processes, and the effects of the amount of parent–child interaction on the structure of the right STG and Verbal Comprehension may be related to alterations in these functions. Previous neuroscientific studies have suggested that the left STG is involved in language comprehension (Hécaen and Albert, 1978) and spoken-word recognition (Howard et al., 1992). The right STG has been implicated in the processing of nonverbal sound discrimination, recognition, and comprehension (McGlone and Young, 1992), processing of linguistic context (Kircher et al., 2001), irony and metaphor comprehension (Eviatar and Just, 2006), and gaze recognition (Akiyama et al., 2006). Both the left and right STG have been shown to be involved in a number of verbal processes (Binder et al., 1994; Herholz et al., 1994; McGuire et al., 1996), and the commonality of the bilateral STG as well as laterality of STG may be important. The key functions of the right STG may be more specialized and focused on the processing of nonliteral information during communicative processes. The effects of the amount of parent–child interaction on the right STG may thus reflect altered processing of nonliteral verbal information among verbal skills, which is also necessary at measures, such as Verbal Comprehension Index, too (Holdnack et al., 2011).
A wide range of evidence has suggested that reductions in STG gray matter structures reflect increased functional integrity of this area during development. Enlarged right STG gray matter structures are observed in children exposed to parental verbal abuse (De Bellis et al., 2002a), mistreated children and adolescents with post-traumatic stress disorder (Tomoda et al., 2011), children with generalized anxiety disorder (De Bellis et al., 2002b), and children and adolescents with autism (Jou et al., 2010). Interestingly, the first three groups are known to have deficits in nonliteral interpersonal ability (Dodge, 1993) and anxiety is also negatively associated with interpersonal ability (Uchiyama et al., 2001), which is relevant to the abovementioned nonliteral information processing during communication. Furthermore, the present study revealed a developmental decrease in rGMD in STG and a reduction in rGMD in areas of the bilateral STG associated with Verbal Comprehension. These data point to the conclusion that the reduction in rGM structures in these areas reflects increased functional integrity. Furthermore, it is tempting to speculate whether the enlarged gray matter structures of STG observed in children who have less parent–child interactions and those observed in the abovementioned clinical groups have common roots (i.e., less or inappropriate social interactions for whatever reason) and the same functional implication (reduced nonliteral communicative processes).
Why the negative correlations between regional gray matter in STG and cognitive functions or pathological processes were seen in the present and previous studies is not clear. However, after the early phase of development, gray matter tends to decrease (Giedd et al., 1999), which is probably caused by synaptic pruning (Sowell et al., 2003). Further, children with superior IQs show the most vigorous cortical thinning in areas, including the temporal gyrus (Shaw et al., 2006). Further, cross-sectionally, more intelligent children generally have a slightly thinner cortex than children with a lower IQ, and this relationship becomes more pronounced with increasing age due to faster developmental cortical thinning in children with higher IQs (Schnack et al., 2014). Particularly, the VCI component has been shown to be specifically and negatively correlated with cortical thickness in the bilateral temporal gyrus (Menary et al., 2013). Thus, one speculation obtained from these studies is the delayed developmental cortical thinning may lead to a relatively larger rGMD in the STG, whereas lower functions form the negative correlation between rGMD and function.
The root cause of the lack of any longitudinal effect of the amount of parent–child interaction on the structures of the left STG is unclear. The number of hours spent with parents did not correlate with longitudinal changes in the gray matter structures of the left STG despite cross-sectional correlations being observed among these structures, the amount of time spent with parents, and verbal knowledge. One simple interpretation is that the amount of parent–child interaction does not affect the structures of the left STG; however, the left STG and its associated functions (verbal and social related) (Takeuchi et al., 2013a, b) may facilitate parent–child interaction. There are also other possibilities, such as the effects of parent–child interactions on the left STG are direct and immediate rather than accumulative; therefore, changes were only detected in cross-sectional analyses. Alternatively, as suggested previously, the effect of parent–child interactions on verbal skills may be most evident during infancy (Harvey, 1999) and may have little impact among children who are of the same age as those in the present sample. Future interventional studies during infancy may be able to solve these questions.
The present study had some limitations. First, this was not an intervention study. Thus, although we have shown that the number of hours spent with parents affects both brain structures and Verbal Comprehension in cross-sectional and longitudinal analyses, we cannot clarify whether spending time with parents has a direct effect on these measures. We effectively ruled out the possibility of a good relationship with the parents or the socioeconomic status of the family having caused these associations by including the relationship with the parents and socioeconomic status as covariates. However, it is possible that unknown related or confounding factors influenced these findings. There are literally numerous factors that affect child cognitive development, and like any relevant psychological studies of parent–child interaction and previous neuroimaging studies of the effects of environmental factors on children's brain development, we did not correct for all of them. One such factor that affects a child's cognitive development that had not been corrected in the analysis is the nature of his or her peers (Harris, 1998). Individual children's tendencies toward spending time with parents or the parents' tendency to willingly interact with children may have directly or indirectly affected these measures. Future intervention studies can describe these issues more precisely. Further, like most large-scale studies of daily habits (Makino, 2007; Taki et al., 2010, 2012b; Przybylski, 2014), we relied on self-report or parental-report measures for daily habits. Although it makes it possible to easily assess what we would like to measure, it may suffer from low reliability compared with objective observations, precise daily recordings, or future studies using these more troublesome but possibly more accurate measures that may provide more precise correlates of parent–child interactions. Furthermore, in this study's questionnaire, duration of time that participants were to consider (e.g., time spent with parents in the past 2 months) was not specified. This procedure (lack of specification of the period time) is common among widely used and established questionnaires for daily habits, such as the morningness–eveningness questionnaire (Horne and Ostberg, 1976), the questionnaire that was used by a Japanese government's study (Makino, 2007), questionnaires that our previous studies used (Taki et al., 2010, 2012b), as well as the questionnaire used by the leading expert of the effects of daily habits in children (Przybylski, 2014). Nevertheless, the lack of specification might introduce unwanted variances.
In conclusion, spending time with parents is directly or indirectly associated with the neurocognitive development of children. Although no definitive causal effects can be reported until intervention studies have been performed, these findings suggest that spending time with parents, particularly that through verbal communication, has beneficial effects on verbal cognition and associated neural development, and guardians of children should consider these effects when they raise children.
Footnotes
This work was supported by JST/RISTEX, JST/CREST, and Ministry of Education, Culture, Sports, Science, and Technology Grant-in-Aid for Young Scientists (B) KAKENHI 23700306. We thank Yuki Yamada for operating the MRI scanner and for being an examiner of psychological tests; the study participants, the other examiners of psychological tests, and all of our colleagues in the Institute of Development, Aging and Cancer and in Tohoku University for their support; and Enago (www.enago.jp) for the English language review.
The authors declare no competing financial interests.
References
- Akiyama T, Kato M, Muramatsu T, Saito F, Nakachi R, Kashima H. A deficit in discriminating gaze direction in a case with right superior temporal gyrus lesion. Neuropsychologia. 2006;44:161–170. doi: 10.1016/j.neuropsychologia.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Azuma H, Ueno K, Fujita K, Maekawa H, Ishikuma T, Sano H. Japanese Wechsler Intelligence Scale for Children. Ed 3. Tokyo: Nihon Bunka Kagakusha; 1998. [Google Scholar]
- Bee HL, Van Egeren LF, Pytkowicz Streissguth A, Nyman BA, Leckie MS. Social class differences in maternal teaching strategies and speech patterns. Dev Psychol. 1969;1:726–734. doi: 10.1037/h0028257. [DOI] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25:60–83. doi: 10.2307/1165312. [DOI] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Yetkin FZ, Jesmanowicz A, Bandettini PA, Wong EC, Estkowski LD, Goldstein MD, Haughton VM. Functional magnetic resonance imaging of human auditory cortex. Ann Neurol. 1994;35:662–672. doi: 10.1002/ana.410350606. [DOI] [PubMed] [Google Scholar]
- Bing E. Effect of childrearing practices on development of differential cognitive abilities. Child Dev. 1963;34:631–648. doi: 10.2307/1126757. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM. The relation of infants' home environments to mental test performance at fifty-four months: a follow-up study. Child Dev. 1976;47:1172–1174. doi: 10.1111/j.1467-8624.1984.tb03817.x. [DOI] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, Flowers L, Wood F, Maldjian JA. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–143. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke-Stewart KA. Interactions between mothers and their young children: characteristics and consequences. Monogr Soc Res Child Dev. 1973;38:1–109. doi: 10.2307/1165725. [DOI] [PubMed] [Google Scholar]
- Culp RE, Watkins RV, Lawrence H, Letts D, Kelly DJ, Rice ML. Maltreated children's language and speech development: abused, neglected, and abused and neglected. First Language. 1991;11:377–389. doi: 10.1177/014272379101103305. [DOI] [Google Scholar]
- De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, Hall J. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol Psychiatry. 2002a;51:544–552. doi: 10.1016/S0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, Birmaher B, Hall J, Moritz G, Ryan ND. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002b;51:553–562. doi: 10.1016/S0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, Hodgins S, Viding E. Size matters: increased grey matter in boys with conduct problems and callous–unemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- Desimone R. Face-selective cells in the temporal cortex of monkeys. J Cogn Neurosci. 1991;3:1–8. doi: 10.1162/jocn.1991.3.1.1. [DOI] [PubMed] [Google Scholar]
- Dodge KA. Social-cognitive mechanisms in the development of conduct disorder and depression. Annu Rev Psychol. 1993;44:559–584. doi: 10.1146/annurev.ps.44.020193.003015. [DOI] [PubMed] [Google Scholar]
- Eviatar Z, Just MA. Brain correlates of discourse processing: an fMRI investigation of irony and conventional metaphor comprehension. Neuropsychologia. 2006;44:2348–2359. doi: 10.1016/j.neuropsychologia.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell RR, Deutscher B. Contributions of receptive vocabulary and maternal style variables to later verbal ability and reading in low-birthweight children. Top Early Child Special Educ. 2002;22:181–190. doi: 10.1177/027112140202200401. [DOI] [Google Scholar]
- Friederici AD, Makuuchi M, Bahlmann J. The role of the posterior superior temporal cortex in sentence comprehension. Neuroreport. 2009;20:563–568. doi: 10.1097/WNR.0b013e3283297dee. [DOI] [PubMed] [Google Scholar]
- Fujita K, Maekawa H, Dairoku H, Yamanaka K. Japanese Wechsler Adult Intelligence Scale. Ed 3. Tokyo: Nihon Bunka Kagakusha; 2006. [Google Scholar]
- Funk JB, Ruppert ES. Language disorders and behavioral problems in preschool children. J Dev Behav Pediatr. 1984;5:357–360. [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Han WJ, Waldfogel J, Brooks-Gunn J. The effects of early maternal employment on later cognitive and behavioral outcomes. J Marriage Family. 2001;63:336–354. doi: 10.1111/j.1741-3737.2001.00336.x. [DOI] [Google Scholar]
- Harris JR. The nurture assumption: why children turn out the way they do. New York: Free; 1998. [Google Scholar]
- Harvey E. Short-term and long-term effects of early parental employment on children of the National Longitudinal Survey of Youth. Dev Psychol. 1999;35:445–459. doi: 10.1037/0012-1649.35.2.445. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hécaen H, Albert ML. Human neuropsychology. New York: John Wiley; 1978. [Google Scholar]
- Henderson D. Contextual specificity, discretion, and cognitive socialization: with special reference to language. Sociology. 1970;4:311–338. [Google Scholar]
- Herholz K, Pietrzyk U, Karbe H, Würker M, Wienhard K, Heiss WD. Individual metabolic anatomy of repeating words demonstrated by MRI-guided positron emission tomography. Neurosci Lett. 1994;182:47–50. doi: 10.1016/0304-3940(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Holdnack J, Goldstein G, Drozdick L. Social perception and WAIS-IV performance in adolescents and adults diagnosed with Asperger's syndrome and autism. Assessment. 2011;18:192–200. doi: 10.1177/1073191110394771. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Howard D, Patterson K, Wise R, Brown W, Friston K, Weiller C, Frackowiak R. The cortical localization of the lexicons: positron emission tomography evidence. Brain. 1992;115:1768–1782. doi: 10.1093/brain/115.6.1769. [DOI] [PubMed] [Google Scholar]
- Ishii H. I want to know this about statistical analysis [Japanese book; Tokeibunseki-no-kokoga-shiritai] Tokyo: Bunko-do; 2005. [Google Scholar]
- Jones PA. Home environment and the development of verbal ability. Child Dev. 1972;43:1081–1086. doi: 10.2307/1127662. [DOI] [Google Scholar]
- Jou RJ, Minshew NJ, Keshavan MS, Vitale MP, Hardan AY. Enlarged right superior temporal gyrus in children and adolescents with autism. Brain Res. 2010;1360:205–212. doi: 10.1016/j.brainres.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh KC, Johnson MH, Dick F, Kadosh RC, Blakemore SJ. Effects of age, task performance, and structural brain development on face processing. Cereb Cortex. 2013;23:1630–1642. doi: 10.1093/cercor/bhs150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara M, Miyashita K, Ohnogi H, Nakazawa J. Psychology manual, methods of questionnaires [Japanese] Kyoto: Kitaohji shobo; 1998. [Google Scholar]
- Kircher TT, Brammer M, Tous Andreu N, Williams SC, McGuire PK. Engagement of right temporal cortex during processing of linguistic context. Neuropsychologia. 2001;39:798–809. doi: 10.1016/S0028-3932(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Kohl HWI, Fulton JE, Caspersen CJ. Assessment of physical activity among children and adolescents: a review and synthesis. Prev Med. 2000;31:S54–S76. doi: 10.1006/pmed.1999.0542. [DOI] [Google Scholar]
- Krishnadas R, McLean J, Batty GD, Burns H, Deans KA, Ford I, McConnachie A, McLean JS, Millar K, Sattar N, Shiels PG, Tannahill C, Velupillai YN, Packard CJ, Cavanagh J. Socioeconomic deprivation and cortical morphology: psychological, social, and biological determinants of ill health study. Psychosom Med. 2013;75:616–623. doi: 10.1097/PSY.0b013e3182a151a7. [DOI] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wüst S, Pruessner JC, Rietschel M, Deuschle M, Meyer-Lindenberg A. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Makino K. Outline and significance of the International Comparative Study on Child Raising and Family Life 2005 [in Japanese] J Natl Womens Educ Center Japan. 2007;11:3–10. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Management and Coordination Agency Headquarters About Juveniles. International comparative report about children and family [in Japanese] Tokyo: Management and Coordination Agency Haj; 1996. [Google Scholar]
- McCartney K. Effect of quality of day care environment on children's language development. Dev Psychol. 1984;20:244–260. doi: 10.1037/0012-1649.20.2.244. [DOI] [Google Scholar]
- McGlone J, Young G. Cerebral localization. In: Baker A, editor. Clinical neurology. Philadelphia: Harper and Row; 1992. [Google Scholar]
- McGuire PK, Silbersweig DA, Frith CD. Functional neuroanatomy of verbal self-monitoring. Brain. 1996;119:907–917. doi: 10.1093/brain/119.3.907. [DOI] [PubMed] [Google Scholar]
- Menary K, Collins PF, Porter JN, Muetzel R, Olson EA, Kumar V, Steinbach M, Lim KO, Luciana M. Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence. 2013;41:597–606. doi: 10.1016/j.intell.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan K, Astington JW, Dack LA. Language and theory of mind: meta-analysis of the relation between language ability and false-belief understanding. Child Dev. 2007;78:622–646. doi: 10.1111/j.1467-8624.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Does amount of time spent in child care predict socioemotional adjustment during the transition to kindergarten? Child Dev. 2003;74:976–1005. doi: 10.1111/1467-8624.00582. [DOI] [PubMed] [Google Scholar]
- O'Connor BP. The search for dimensional structure differences between normality and abnormality: a statistical review of published data on personality and psychopathology. J Pers Soc Psychol. 2002;83:962–982. doi: 10.1037/0022-3514.83.4.962. [DOI] [PubMed] [Google Scholar]
- Ohyama N. The second basic report about juveniles' life and minds. Tokyo: Printing Bureau, Ministry of Finance; 2001. Parent–child relationship and parents' influence. [Google Scholar]
- Okamoto M. Attempts to connect body and minds, Vol II [in Japanese] Bull Toyohashi Sozo Junior College. 2002:61–74. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parcel TL, Menaghan EG. Maternal working conditions and children's verbal facility: studying the intergenerational transmission of inequality from mothers to young children. Soc Psychol Q. 1990;53:132–147. doi: 10.2307/2786675. [DOI] [Google Scholar]
- Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- Przybylski AK. Electronic gaming and psychosocial adjustment. Pediatrics. 2014;134:e716–e722. doi: 10.1542/peds.2013-4021. [DOI] [PubMed] [Google Scholar]
- Ramey CT, Farran DC, Campbell FA. Predicting IQ from mother-infant interactions. Child Dev. 1979;50:804–814. doi: 10.2307/1128947. [DOI] [PubMed] [Google Scholar]
- Ramsden S, Richardson FM, Josse G, Thomas MS, Ellis C, Shakeshaft C, Seghier ML, Price CJ. Verbal and non-verbal intelligence changes in the teenage brain. Nature. 2011;479:113–116. doi: 10.1038/nature10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JP. The validity and reliability of diaries versus alternative time use measures. In: Juster FT, Stafford FP, editors. Time, goods, and well-being. Ann Arbor, MI: Institute for Social Research, University of Michigan; 1985. pp. 63–92. [Google Scholar]
- Ruhm CJ. Parental employment and child cognitive development. J Hum Resources. 2004;39:155–192. doi: 10.2307/3559009. [DOI] [Google Scholar]
- Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Pol HEH. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex. 2014 doi: 10.1093/cercor/bht357. doi: 10.1093/cercor/bht357. Advance online publication. Retrieved Jan 9, 2014. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Silver M, Montana G, Nichols TE. False positives in neuroimaging genetics using voxel-based morphometry data. Neuroimage. 2011;54:992–1000. doi: 10.1016/j.neuroimage.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. Mahway, NJ: Lawrence Erlbaum; 1996. pp. 366–367. [Google Scholar]
- Sucksmith E, Allison C, Baron-Cohen S, Chakrabarti B, Hoekstra RA. Empathy and emotion recognition in people with autism, first-degree relatives, and controls. Neuropsychologia. 2013;51:98–105. doi: 10.1016/j.neuropsychologia.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi O, Toguchi Y. Light and shadow of KIZUNA: a construction of KIZUNA scale (Sunnyside and darkside of social bond) [in Japanese] Bull Faculty Soc Kansai Univ. 2006;37:3–28. [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. Brain structures associated with executive functions during everyday events in a non-clinical sample. Brain Struct Funct. 2013a;218:1017–1032. doi: 10.1007/s00429-012-0444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Thyreau B, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R. White matter structures associated with empathizing and systemizing in young adults. Neuroimage. 2013b;77:222–236. doi: 10.1016/j.neuroimage.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Asano K, Asano M, Sassa Y, Yokota S, Kotozaki Y, Nouchi R, Kawashima R. The impact of television viewing on brain structures: cross-sectional and longitudinal analyses. Cereb Cortex. 2013c doi: 10.1093/cercor/bht315. doi: 10.1093/cercor/bht315. Advance online publication. Retrieved Nov 20, 2013. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Hashizume H, Sassa Y, Sekiguchi A, Kotozaki Y, Nakagawa S, Nagase T, Miyauchi CM, Kawashima R. Anatomical correlates of quality of life: evidence from voxel-based morphometry. Hum Brain Mapp. 2014;35:1834–1846. doi: 10.1002/hbm.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Sassa Y, Takeuchi H, Asano M, Asano K, Kawashima R. Breakfast staple types affect brain gray matter volume and cognitive function in healthy children. PLoS One. 2010;5:e15213. doi: 10.1371/journal.pone.0015213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Sassa Y, Takeuchi H, Asano M, Asano K, Kotozaki Y, Nouchi R, Wu K, Fukuda H. Correlation among body height, intelligence, and brain gray matter volume in healthy children. Neuroimage. 2012a;59:1023–1027. doi: 10.1016/j.neuroimage.2011.08.092. [DOI] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Thyreau B, Sassa Y, Takeuchi H, Wu K, Kotozaki Y, Nouchi R, Asano M, Asano K. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. Neuroimage. 2012b;60:471–475. doi: 10.1016/j.neuroimage.2011.11.072. [DOI] [PubMed] [Google Scholar]
- Toguchi Y. Public lectures: the role of Japanese bonds on human relations: focusing on the bonds among married couples [in Japanese] Kansei: Kansei University's Research Institute of Politic and Economy; 2009. Seminar Annual Report 83–90. [Google Scholar]
- Tomoda A, Sheu YS, Rabi K, Suzuki H, Navalta CP, Polcari A, Teicher MH. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage. 2011;54:S280–S286. doi: 10.1016/j.neuroimage.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulkin SR, Kagan J. Mother–child interaction in the first year of life. Child Dev. 1972;43:31–41. doi: 10.2307/1127869. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uchiyama K, Shimai T, Utsuki N, Otake K. EQS manual (Practical Education) Tokyo: Jitsumukyoiku Syuppan; 2001. [Google Scholar]
- Ullman H, Almeida R, Klingberg T. Structural maturation and brain activity predict future working memory capacity during childhood development. J Neurosci. 2014;34:1592–1598. doi: 10.1523/JNEUROSCI.0842-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé P, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G, Milovan D, Paus T, Rumsey J. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Lee NR, Prom-Wormley EC, Medland SE, Lenroot RK, Clasen LS, Schmitt JE, Neale MC, Giedd JN. A bivariate twin study of regional brain volumes and verbal and nonverbal intellectual skills during childhood and adolescence. Behav Genet. 2010;40:125–134. doi: 10.1007/s10519-009-9329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]