Figure 6.
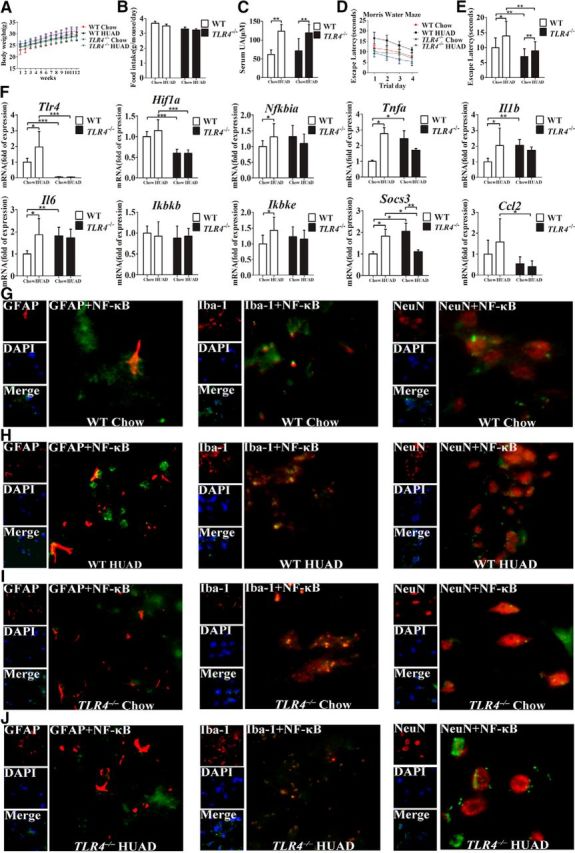
TLR4 loss ameliorates the impaired cognitive dysfunction and reduces hippocampal inflammation induced by a HUAD. A, B, The characteristics of TLR4−/ − and WT mice fed a HUAD for 12 weeks. A, Body weight. B, Food consumption. C, Time course of serum UA concentration after the onset of HUAD feeding (Student's t tests, **p < 0.01, n = 6 rats per group). D, E, Spatial learning of the mice after the onset of HUAD feeding, as demonstrated in the test of Morris water maze. Each trial presented was the average of four individual tests, and four trials were performed with the escape latency to the platform being recorded (Student's t tests, *p < 0.05; **p < 0.01, n = 6 rats per group). F, Expression levels of inflammatory mediators, including proinflammatory cytokines (Il6, Il1b, Tnfa, Socs3, and Ccl2) and TLR4/NF-κB signaling (Tlr4, Nfkbia, Ikbkb, and Ikbke), were determined by qRT-PCR in hippocampi of mice fed a HUAD for 12 weeks (Student's t tests, *p < 0.05; **p < 0.01; ***p < 0.001, n = 6 rats per group). G–J, Hippocampal tissues were immunostained for GFAP and RelA, Iba-1 and RelA, and NeuN and RelA (400×) after 12 weeks of being fed a HUAD. RelA was used for reporting on NF-κB. DAPI nuclear staining revealed all cells in the section. WT Chow (G), WT HUAD (H), TLR4−/− Chow (I), and TLR4−/− HUAD (J). GFAP, DAPI, Merge (DAPI + NF-κB), GFAP + NF-κB; Iba-1, DAPI, Merge (DAPI + NF-κB), Iba-1 + NF-κB; and NeuN, DAPI, Merge (DAPI + NF-κB), and NeuN + NF-κB. All displayed values are the mean ± SEM.
