Abstract
Objective
Although feeding problems are a common concern in children with autism spectrum disorder (ASD), few longitudinal studies have examined their persistence over time. The purpose of this study was to examine the developmental progression of feeding problems across four time points in preschoolers with ASD.
Methods
Group-based trajectory analyses revealed four distinct trajectories of feeding problems in our sample (N = 396).
Results
The majority of children showed levels of feeding problems that were low from the outset and stable (Group 1; 26.3%) or moderate and declining over time (Group 2; 38.9%). A third group (26.5%) showed high levels of feeding problems as preschoolers that declined to the average range by school age. Few participants (8.3%) showed evidence of severe chronic feeding problems. Feeding problems were more highly correlated with general behavior problems than with autism symptom severity.
Conclusions
Overall, our findings demonstrated that in our sample of children with ASD, most feeding problems remitted over time, but a small subgroup showed chronic feeding problems into school age. It is important to consider and assess feeding problems in ASD against the backdrop of typical development, as many children with ASD may show improvement with age.
Keywords: autism spectrum disorder, feeding problems, food selectivity, preschoolers
Introduction
Autism spectrum disorder (ASD) is often associated with various comorbid conditions including anxiety, sleep difficulties, gastrointestinal symptoms, and feeding problems (Kozlowski, Matson, Belva, & Rieske, 2012; Mannion & Leader, 2013; Muskens, Velders, & Staal, 2017). The term “feeding problems” encompasses a range of concerns, such as food selectivity (“picky eating”), problematic mealtime behavior, and oral/motor difficulties. Feeding problems stand out as a particular concern in children with ASD due to their high-reported prevalence; Ledford and Gast (2006) found prevalence rates of 46–89% in a literature review. Sharp et al. (2013) found that children with ASD are five times more likely to have a feeding problem than typically developing children, and other studies found that children with ASD show higher rates of nutritional deficiencies (Bandini et al., 2010; Liu et al., 2016). Furthermore, parents of children with feeding problems report struggling to manage their children’s diets and nutrient intake, and increased mealtime stress (Rogers, Magill-Evans, & Rempel, 2012; Suarez, Atchison, & Lagerway, 2014). Thus, feeding problems of children with ASD have significant ramifications for their health, development, and psychosocial environment.
Given the impact of feeding problems in children with ASD, it is important to understand how these problems change and/or persist over time. In typically developing children, feeding problems are often believed to be transient. For example, Cano et al. (2015) examined food selectivity from ages 1.5 to 6 years and found that 55% of typically developing children were never picky eaters, 32% showed a remitting pattern, 4% had late-onset (i.e., only at age six years) picky eating, and 4% showed persistent food selectivity. However, Kuschner et al. (2015) suggested that feeding problems may persist in ASD given symptoms such as sensory sensitivities and insistence on sameness (American Psychiatric Association, 2013). Cross-sectional studies examining a range of feeding problems in children with ASD have produced mixed results, with some showing that feeding problems decrease with age (Beighley, Matson, Rieske, & Adams, 2013), and others showing no relationship with age (Bandini et al., 2010; Williams, Gibbons, & Schreck, 2005).
To our knowledge, only two studies have examined feeding problems in children with ASD using longitudinal designs. Suarez, Nelson, and Curtis (2014) measured food selectivity in children with ASD (initial N = 141) at two time points (20 months apart) using randomly selected participants from an online database. Children’s ages ranged from 3.4 to 8.9 years at time 1 (M = 6.6), and from 4.6 to 10.7 years (M = 8.3) at time 2. They found no changes in levels of food selectivity and 60.1% of children fell into the same food selectivity category at both times. Bandini et al. (2017) also examined feeding problems in a community sample of children with ASD (initial N = 53) across two time points. On average, children were 6.8 years old (SD = 2.3) at time 1, and 13.2 years old (SD = 2.5) at time 2. They found that food refusal and problematic mealtime behavior decreased significantly, whereas children’s food repertoires remained largely the same. However, they noted considerable variability among participants, with more than half remaining highly selective. Furthermore, both longitudinal studies experienced substantial (more than 60%) attrition. Evidently, more research is needed to understand the prevalence and persistence of feeding problems in children with ASD.
In addition to trajectories, it is also important to examine covariates and key risk factors of feeding problems in this population. Sensory sensitivity, internalizing behavior, and externalizing behavior have all been associated with feeding problems in children with ASD in multiple cross-sectional studies (Postorino et al., 2015; Suarez et al., 2014). Some studies have reported that autism symptoms, cognitive abilities, and adaptive functioning are related to the level of feeding problems in ASD; other studies found no such associations (Allen et al., 2015; Johnson et al., 2014; Kuschner et al., 2015; Postorino et al., 2015). Thus, more research is needed to clarify risk factors and covariates of feeding problems in ASD, and to determine how these relations might change over time.
The purpose of the current study was to examine the developmental progression of feeding problems in a large cohort of preschoolers with ASD. We used group-based trajectory analyses to determine the number and shape of distinct trajectories of feeding problems. A secondary objective was to understand predictors of trajectory group membership, as well as temporal relationships between feeding problems and covariates (e.g., other maladaptive behavior). We expected to identify a minimum of two distinct trajectories. We anticipated that some children would show high levels of feeding problems that were stable over time, whereas others would show decreasing levels of problems over time. Moreover, we hypothesized that general behavior problems would be associated with feeding problems over time.
Methods
Participants and Procedure
Participants were enrolled in the longitudinal Pathways in ASD study (Szatmari et al., 2015) and recruited from five centers in Canada: Halifax, Nova Scotia; Montreal, Quebec; Hamilton, Ontario; Edmonton, Alberta; and the Greater Vancouver/Fraser Valley regions of British Columbia. Research ethics boards at each site approved the study protocol, and all families provided informed consent. All participants were between 24 and 60 months old at recruitment and had a recent (i.e., within 4 months) diagnosis of ASD confirmed by the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000), the Autism Diagnostic Interview Revised (Rutter et al., 2003), and expert clinician judgment. Exclusion criteria were cerebral palsy or other neuromotor disorder interfering with study assessments, known genetic or chromosomal abnormality, and severe visual or hearing impairment. Only one child per family participated to ensure independence of observations.
Only participants with data from the Behavioral Pediatrics Feeding Assessment Scale (BPFAS; Crist et al., 1994; Crist & Napier-Phillips, 2001) for at least one of the time points of interest were included. The analytic sample comprised 396 children (334 boys) with an average age of 38.3 months (SD = 8.8) at the time of diagnosis. The mean intellectual ability and adaptive behavior scores in our sample were similar to those found in other community studies of children diagnosed with ASD as preschoolers (e.g., Dietz, Swinkels, Buitelaar, van Daalen, & van Engeland, 2007; Rivard, Terroux, Mercier, & Parent-Boursier, 2014). Parent respondents were most frequently biological mothers (86.1%). At the first time point (T1, within 4 months of diagnosis), most parent respondents were married or had a common law partner (84.0%), had at least some postsecondary education (86%), and identified themselves as “white” (70.5%). Their annual household incomes varied, with 57.4% under $80,000 (Canadian), and 35.6% at or above $80,000 (6.8% missing/preferred not to answer).
This analysis used data from four time points. Time 1 (T1; M = 2.6 months after diagnosis, SD = 2.5) occurred when participants were a mean of 40.9 months old (SD = 9.3), Time 2 was approximately six months later (T2; Mage = 47.8 months, SD = 9.5), and Time 3 was approximately six months after T2 (T3; Mage = 54.0 months, SD = 9.2). Time 4 took place at or near age six (T4; Mage = 79.2 months, SD = 3.7), after about one year of school. Since age at diagnosis varied, the T3–T4 interval also varied. Of the 396 children included in this study, BPFAS data were available for 374 (94%) at T1, 335 (85%) at T2, 317 (80%) at T3, and 270 (68%) at T4; 226 children had BPFAS data for all four time points. A series of Welch’s independent sample t-tests was conducted comparing children with and without BPFAS data at each time point on sex and age at diagnosis, as well as T1 intellectual ability, ASD symptom severity, household income, and parental education. If income or education data were missing for T1, we imputed data from T2 or T3 if possible, as T1–T3 occurred over approximately one year. Using the Benjamini and Hochberg (1995) procedure to control the false discovery rate across multiple comparisons, no significant differences were observed between participants with and without missing BPFAS data. This provided reasonable evidence that data were missing at random.
Most assessments took place in university or hospital research centers, with some in children’s homes, childcare centers, or schools. Standardized measures were administered by psychometrists or research assistants trained and supervised by psychologists. Parents completed questionnaires at home or in the clinic/lab, and interviews were conducted with a primary caregiver in person or by telephone.
Measures
Behavioral Pediatrics Feeding Assessment Scale
The BPFAS is a parent-report measure of mealtime and feeding behavior associated with poor nutritional intake (Crist et al., 1994; Crist & Napier-Phillips, 2001). Unlike other questionnaires focused on one type of feeding problem (e.g., food selectivity), the BPFAS is a comprehensive measure that assesses behavioral and skill-based feeding problems. It includes 35 items; the first 25 describe the child’s behavior, skills, and food selectivity (e.g., enjoys eating, has problems chewing foods), and the last 10 items (not used in this study) describe the parent’s feelings about and strategies for managing feeding problems. For each item, parents respond how frequently the behavior occurs (using Likert scale: 1 = never to 5 = always). Items are phrased either positively or negatively, and positive items are reverse-scored. We used frequency scores for the 25 child feeding items. Possible total scores range from 25 to 125, such that greater scores indicate higher levels of feeding problems. Dovey, Jordan, Aldridge, and Martin (2013) established that a BPFAS cutoff score of 61 discriminated between clinical and non-clinical samples with 87% accuracy. Allen et al. (2015) previously examined the factor structure and validity of the 25 BPFAS child feeding items in this cohort of preschoolers and found support for a three-factor model of feeding problems (food acceptance, medical oral/motor, and mealtime behavior). We generated scores for each of these factors. Mean factor scores were computed ranging from 1 to 5, with higher scores indicating more problems. Previous research with the BPFAS has shown that the child feeding items have adequate internal consistency (Cronbach’s α = .84; Crist et al., 1994), and has validated use of these items in populations including children with cystic fibrosis (Crist et al., 1994), diabetes (Patton, Dolan, & Powers, 2006), and children who are overweight or obese (Davis, Canter, Stough, Gillette, & Patton, 2014).
Autism Diagnostic Observation Schedule
The ADOS is a semi-structured assessment of ASD symptoms that uses planned social and play activities to elicit behavior relevant to ASD diagnosis (Lord et al., 2000). The child’s behavior is coded to indicate levels of restricted/repetitive behavior and impairment in social communication. The autism severity metric (Gotham, Pickles, & Lord, 2009), used in the current study, ranges from 1 to 10, with higher scores indicating more severe ASD symptoms. The ADOS has been shown to have good interrater reliability and test-retest reliability, and excellent internal consistency (Lord et al., 2000).
Merrill-Palmer-Revised Scales of Development
The Merrill-Palmer-Revised Scales of Development (M-P-R) is an individually administered, predominantly non-verbal measure of developmental functioning in infants and children from ages 1 month to 6 years, 6 months (Roid & Sampers, 2004). The overall developmental index (DI) standard score (M = 100, SD = 15) was used in the current study. The DI demonstrates adequate reliability (internal consistency of .97–.98, test-retest reliability of .89), is correlated with other measures of developmental functioning (e.g., .92 with the Bayley Scales of Infant and Toddler Development), and is sensitive to developmental delays in children with ASD (Dempsey et al., 2018).
Vineland Adaptive Behavior Scales, Second Edition
The Vineland Adaptive Behavior Scales, Second Edition (VABS-II) Survey Form is a semi-structured interview measure of adaptive functioning from birth to age 90 (Sparrow, Cicchetti, & Balla, 2005). It employs open-ended questions to assess children’s levels of communication, daily living skills, socialization, and motor skills. We used Adaptive Behavior Composite (ABC) standard scores (M = 100, SD = 15). The VABS-II demonstrates adequate reliability (e.g., internal consistency of .94, test-retest reliability of .88, and interrater reliability of .74 for the ABC), and has shown moderate to high correlations with other adaptive behavior measures (e.g., r = .78 between the VABS-II ABC and the Adaptive Behavior Assessment System, Second Edition; Harrison & Oakland, 2000; Sparrow et al., 2005). It is often used to assess adaptive functioning in children with ASD (e.g., McDonald et al., 2015).
Children’s Sleep Habits Questionnaire
The Children’s Sleep Habits Questionnaire (CSHQ) is a widely used parent-report questionnaire that screens for sleep problems in 2- to 10-year-olds (Goodlin-Jones, Sitnick, Tang, Liu, & Anders, 2008). The CSHQ consists of 33 scorable items. For each item, parents respond using a Likert scale (rarely = 0–1 nights per week to usually = 5–7 nights per week). These items are summed to provide the CSHQ Total score used in this study, ranging from 33 to 99, with greater scores indicating higher levels of sleep problems. The CSHQ has demonstrated adequate internal consistency (Cronbach’s α = .68 for community samples, .78 for clinical samples) and acceptable test-retest reliability (.62–.79; Owens, Spirito, & McGuinn, 2000). The CSHQ is frequently used as a measure of sleep problems in children with ASD (e.g., Honomichl, Goodlin-Jones, Burnham, Gaylor, & Anders, 2002).
Child Behavior Checklist
The Child Behavior Checklist (CBCL) for ages 1.5–5 years is a widely used, norm-referenced tool for measuring externalizing and internalizing behavior in young children (Achenbach & Rescorla, 2000). Parents indicate their children’s problem behavior (e.g., can’t sit still, cries a lot) within the past two months on 99 items using a scale from 0 (not true) to 2 (very true or often true). Standard scores (T-scores, M = 50, SD = 10) for the internalizing and externalizing subscales were used in this study. The CBCL has shown good internal consistency (Cronbach’s α = .80 for internalizing subscale, .90 for externalizing subscale) and factor validity in young children with ASD (Pandolfi, Magyar, & Dill, 2009).
Repetitive Behavior Scale Revised
The Repetitive Behavior Scale Revised (RBS-R) is a parent-report measure of the restricted and repetitive behaviors characteristic of ASD, including stereotyped, self-injurious, compulsive, ritualistic, sameness, and restricted behavior (Bodfish, Symons, Parker, & Lewis, 2000). Parents rate their children’s behavior across 43 items using a scale from 0 (behavior does not occur) to 3 (behavior occurs and is a severe problem). The total score used in the current analysis has a possible range of 0 to 129, with greater scores indicating higher levels of repetitive behavior. The RBS-R has demonstrated good internal consistency (Cronbach’s α for all subscales ≥ .78), interrater reliability (.70 for an outpatient sample), and factor validity (Lam & Aman, 2007). Furthermore, Mirenda et al. (2010) validated the RBS-R in this cohort of preschoolers with ASD.
Income
Parent respondents indicated their family income using an 11-point Likert scale. Response options ranged from 1 (<$5,000 Canadian) to 11 (>$80,000). Responses were grouped into two categories: less than $80,000, and $80,000 and above.
Data Analysis
Semi-parametric group-based modeling (Nagin, 1999) was used to identify latent sub-groups with similar patterns of change (trajectories) in BPFAS frequency scores of the child feeding items from T1 to T4. Trajectory models were estimated in PROC TRAJ for SAS (version 9.4), using maximum likelihood estimation. PROC TRAJ assumes that missing data are missing at random. The output of each model includes probabilities of group membership (πj) and an estimated trajectory curve (up to a fourth order polynomial) for each group. Following Kass and Raferty (1995), we used the Bayesian Information Criterion (BIC) to select the best-fitting model (selecting maximum BIC, i.e., least negative value). We evaluated model adequacy by examining the average group posterior probability for each group (AvePPj >0.7), the odds of correct classification (OCCj >5), and the correspondence between estimated group probability (πj) and proportion of participants assigned to each group (Pj; Nagin, 1999). Finally, we also considered model parsimony and theoretical clarity. Descriptive statistics for the BPFAS factor scores (Allen et al., 2015) were calculated to characterize each trajectory group.
After identifying the best model of BPFAS trajectory groups, we examined risk factors and covariates of trajectory group membership. Several T1 variables were included in the model to examine associations with group membership: age at diagnosis, sex, household income, intellectual ability (M-P-R DI standard score), and ASD symptom severity (ADOS severity metric). Other variables (for which data were collected at all four time points) were included as time-varying covariates to examine associations with feeding problems over time. These were repetitive behavior (RBS-R Total score), sleep difficulties (CSHQ Total score), adaptive functioning (VABS-II ABC), externalizing behavior (CBCL externalizing T-score), and internalizing behavior (CBCL internalizing T-score).
Results
Developmental Trajectories
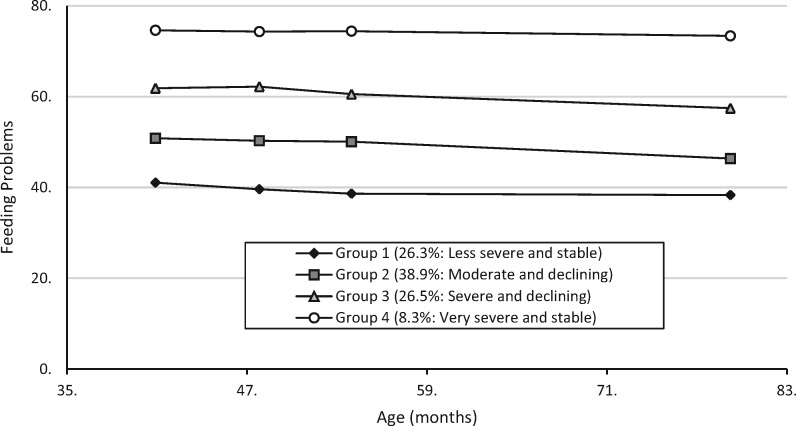
Fit indices for the tested models of feeding problem trajectories are displayed in Table I. Figure 1 shows the four-group solution with linear trajectories that we selected as the best model of the data. Adequacy statistics for this model (see Supplementary Table 1) showed that average group posterior probabilities and odds of correct classification were above conventional standards (0.7 and 5, respectively), and the ratios between estimated probability of group membership and proportion of participants assigned to each group were all close to 1. Group 1 (n = 104) showed a stable trajectory of less severe feeding problems that were well below the clinical cutoff score of 61(Dovey et al., 2013; intercept = 41.18, SE = 0.90; slope = −0.04, SE = 0.02), and was labeled as “less severe and stable.” Group 2 (n = 154) showed symptoms that were more severe than in Group 1 but still consistently below the clinical cutoff, with a small but statistically significant decline in feeding problems over time (intercept = 52.96, SE = 1.00; slope = −0.11, SE = 0.02). Group 2 was labeled as “moderate and declining.” Group 3 (n = 105) showed feeding problems that were, on average, above the clinical cutoff at T1 but below cutoff by T4, showing a small but statistically significant decline (intercept = 63.92, SE = 1.89; slope = −0.10, SE = 0.03). Group 3 was labeled as “severe and declining.” Lastly, Group 4 (n = 33), labeled “very severe and stable,” showed a stable trajectory of feeding problems scores well above the clinical cutoff score (intercept = 72.53, SE = 1.80; slope = 0.04, SE = 0.07).
Table I.
Fit Statistics for Trajectory Models of Feeding Problems in Children with ASD
| Model | Bayesian information criterion |
|---|---|
| 1 group linear | −5089.08 |
| 2 groups linear | −4832.11 |
| 3 groups linear | −4746.03 |
| 4 groups lineara | −4716.54 |
| 5 groups linear | −4719.04 |
| 3 groups quadratic | −4746.71 |
| 4 groups quadratic | −4720.74 |
| 5 groups quadratic | −4726.86 |
| 3 groups polynomial | −4751.82 |
| 4 groups polynomial | −4722.33 |
| 5 groups polynomial | −4730.83 |
Note. ASD = autism spectrum disorder.
Best-fitting model.
Figure 1.
Developmental trajectories of feeding problems in children with autism. Curves are based on frequency scores for the child behavior items on the Behavioral Pediatrics Feeding Assessment Scale (BPFAS; Crist & Napier-Phillips, 2001). Total N = 396.
Descriptive statistics for the BPFAS factor scores (food acceptance, medical oral/motor, and mealtime behavior) by trajectory group and assessment interval are shown in Supplementary Table 2. In general, scores appeared lower on the medical oral/motor factor than the food acceptance and mealtime behavior factors. However, all factors followed similar patterns across trajectory groups, such that they were lowest in the less severe and stable group (Group 1), and highest in the very severe and stable group (Group 4).
Predictors and Covariates
Descriptive statistics for the potential T1 predictors of trajectory group membership are displayed in Supplementary Table 3. We examined how each predictor influenced the likelihood of belonging to each trajectory group, relative to the reference group (Group 1, less severe and stable). Most T1 predictors were not associated with group membership (see Table II). The only significant finding was that the categorical income variable showed a negative relationship with Group 3 membership. Specifically, participants with higher income ($80,000 or more) were more likely to be in Group 1 (less severe and stable) than Group 3 (severe and declining) relative to participants with lower income (less than $80,000).
Table II.
Impact of Predictors on the Likelihood of Belonging to Each Trajectory Group, Relative to the Reference Group (Group 1, Less Severe and Stable)
| Trajectory groups | Risk factor | Coefficient | SE | P |
|---|---|---|---|---|
| Group 2 (moderate and declining) | Age at diagnosis (months) | −0.00 | 0.03 | .864 |
| Sex | −0.50 | 0.51 | .327 | |
| Income | −0.41 | 0.42 | .324 | |
| M-P-R DI standard score | −0.00 | 0.01 | .686 | |
| ADOS severity metric | −0.23 | 0.12 | .057 | |
| Group 3 (severe and declining) | Age at diagnosis (months) | 0.02 | 0.03 | .581 |
| Sex | −0.85 | 0.59 | .151 | |
| Income | −1.00 | 0.46 | .029 | |
| M-P-R DI standard score | 0.01 | 0.01 | .300 | |
| ADOS severity metric | 0.09 | 0.15 | .533 | |
| Group 4 (very severe and stable) | Age at diagnosis (months) | 0.03 | 0.04 | .536 |
| Sex | −10.13 | 107.96 | .925 | |
| Income | −0.33 | 0.68 | .630 | |
| M-P-R DI standard score | 0.00 | 0.01 | .7866 | |
| ADOS severity metric | −0.32 | 0.19 | .091 |
Note. M-P-R DI = Merrill-Palmer-Revised Scales of Development, Developmental Index; ADOS = Autism Diagnostic Observation Schedule.
A positive coefficient indicates a higher likelihood of being in the non-reference trajectory group.
Descriptive statistics for the time-varying covariates are shown in Supplementary Table 4. These results indicate associations with feeding problems over time within each trajectory group (see Table III). Internalizing symptoms were significantly positively associated with feeding problems in all four trajectory groups. As internalizing symptoms increased over time, so did feeding problems. Associations with other variables differed according to trajectory group. Repetitive behavior was positively associated with feeding problems in Groups 2 (moderate and declining) and 3 (severe and declining). Sleep difficulties were only associated with feeding problems in Group 2, for which they showed a positive relationship. Adaptive behavior was significantly but differently associated with feeding problems in Groups 2 and 4. Adaptive behavior was negatively associated with feeding problems in Group 2 (moderate and declining), such that as adaptive behavior increased over time, the level of feeding problems decreased. Interestingly, however, adaptive behavior was positively associated with feeding problems in Group 4 (very severe and stable), such that increased feeding problems were associated with higher levels of adaptive behavior across time. Finally, externalizing symptoms were only associated with feeding problems in Group 4, for which the relation was significant and positive.
Table III.
Associations Between Time-Varying Covariates and Feeding Problems According to Trajectory Group Membership
| Trajectory groups | Predictor | Coefficient | SE | p |
|---|---|---|---|---|
| Group 1 (less severe and stable) | CSHQ total score | 0.05 | 0.08 | .511 |
| VABS-II ABC | −0.04 | 0.04 | .324 | |
| CBCL internalizing | 0.24 | 0.07 | .001 | |
| CBCL externalizing | 0.06 | 0.06 | .323 | |
| RBS-R total score | 0.08 | 0.04 | .057 | |
| Group 2 (moderate and declining) | CSHQ total score | 0.23 | 0.05 | <.001 |
| VABS-II ABC | −0.12 | 0.04 | <.001 | |
| CBCL internalizing | 0.19 | 0.07 | .005 | |
| CBCL externalizing | 0.11 | 0.06 | .077 | |
| RBS-R total score | 0.09 | 0.04 | .012 | |
| Group 3 (severe and declining) | CSHQ total score | 0.14 | 0.09 | .105 |
| VABS-II ABC | −0.03 | 0.05 | .531 | |
| CBCL internalizing | 0.23 | 0.08 | .006 | |
| CBCL externalizing | 0.13 | 0.08 | .088 | |
| RBS-R total score | 0.17 | 0.05 | <.001 | |
| Group 4 (very severe and stable) | CSHQ total score | −0.02 | 0.13 | .861 |
| VABS-II ABC | 0.21 | 0.08 | .005 | |
| CBCL internalizing | 0.27 | 0.14 | .044 | |
| CBCL externalizing | 0.69 | 0.15 | <.001 | |
| RBS-R total score | 0.08 | 0.06 | .229 |
Note. RBS-R = Repetitive Behavior Scale-Revised; CSHQ = Children’s Sleep Habits Questionnaire; VABS-II ABC = Vineland Adaptive Behavior Scales, Second Edition, Adaptive Behavior Composite; CBCL = Child Behavior Checklist.
A positive coefficient indicates that as feeding problems increase/decrease over time, so does the covariate.
Discussion
The purpose of this study was to elucidate patterns of feeding problems in young children with ASD, and the predictors and covariates of persistent feeding problems. Participants displayed notable heterogeneity in the patterns of feeding problems over time, showing four distinct trajectories. In spite of this variability, most children (65.2%) showed feeding problems that were either low from the outset (Group 1) or moderate (but still below clinical cutoffs) and declining over time (Group 2). Group 3 (26.5%) showed clinically significant levels of feeding problems as preschoolers that declined to the typical range by school entry. Only a small subgroup of children in this cohort (8.3%) showed evidence of clinically significant and chronic feeding problems. The developmental trajectories we found are similar to the patterns of some feeding problems in typically developing children. For example, Cano et al. (2015) found that 87% of typically developing children had either low or declining levels of food selectivity, and only 8% still had high levels of food selectivity at age 6 (either persistent since preschool, or newly onset since age three years). Food selectivity is only one type of feeding problem, whereas our study also measured feeding skills and mealtime behavior. However, similar to typically developing children, it appears as though feeding problems remit over time in most children with ASD. Furthermore, BPFAS scores in our sample for children with (i.e., Group 4) and without (i.e., Groups 1 and 2) significant feeding problems were similar to those found in typically developing children (Dovey et al., 2013). Overall, the results of our trajectory analysis indicate that in the majority of children with ASD who have feeding problems, these decrease over time and are below clinical cutoffs by school age.
Predictors
In addition to trajectories, we examined predictors and covariates of feeding problems in children with ASD. In the current study, most potential predictors did not significantly predict trajectory group membership, and the relations found were not readily interpretable. For example, lower family income predicted membership in Group 3 (severe and declining) over Group 1. However, no relationships were found for any other groups, so we cannot draw strong conclusions about the relationship between income and feeding problems. In general, it appears that prediction of which children will have long-standing difficulty with feeding problems is poor based on variables assessed around time of diagnosis.
Covariates
Despite limited ability to predict trajectory group membership, associations with relevant variables over time are also important for characterizing feeding problems in ASD. For all four trajectory groups, internalizing behavior problems were positively associated with feeding difficulties over time. This is both logical and consistent with previous research (e.g., Allen et al., 2015; Johnson et al., 2014; Postorino et al., 2015). Children with higher levels of anxiety or mood difficulties may be more apprehensive about trying new foods. Associations with other variables differed according to trajectory group. For example, feeding problems were positively associated with sleep difficulties and negatively associated with adaptive behavior in Group 2 (moderate and declining), and positively associated with repetitive behavior in both Groups 2 and 3 (severe and declining). Generally, this seems to suggest that children who experience behavioral challenges show effects in multiple domains, one of which is eating/feeding. This is consistent with findings concerning feeding problems in typically developing children. Winsper and Wolke (2014) found that feeding problems were related to dysregulated behavior across childhood. Altogether, it appears that feeding problems in our sample of children with ASD were associated with other difficulties with regulation (i.e., sleep disturbances, internalizing behavior), rather than severity of ASD symptoms. That is, feeding problems are often only part of a constellation of behavioral challenges in children with ASD. This has implications for intervention; treating these associated problems could lead to improvement in feeding problems in children with ASD—and the converse might also be true.
While feeding problems were related to other behavioral challenges across all trajectory groups, it is especially important to determine any characteristics that distinguish children with the most severe problems. A unique association shown by children in the most severe trajectory group (Group 4) was a positive relation between externalizing behavior and feeding problems over time. Thus, for children with the most severe and chronic feeding problems, levels of disruptive behavior varied in tandem with feeding problems over time. This could indicate that poor diet or nutritional inadequacy leads to more disruptive behavior, or that feeding problems are one symptom of a severe and pervasive behavioral disorder. Alternatively, global factors (e.g., coercive family processes; Lucyshyn et al., 2015) may influence both disruptive behavior and feeding problems in a similar fashion. Other factors may be individual; the capacity for self-regulation is often thought to influence multiple domains of functioning (Moffitt et al., 2011). Regardless, the important message is that feeding problems are generally associated with behavioral challenges in other areas, and children who show feeding problems with externalizing symptoms may be at particularly at risk for a chronic course with negative effects on families. Interventions for these children may be more successful if they address both feeding problems and externalizing behavior, or examine global factors contributing to both problems.
Limitations and Future Research
The strengths of this study include the large sample size and the use of an inception cohort. To our knowledge, this is the largest longitudinal study to date examining feeding problems in children with ASD. Despite its strengths, the present study has some limitations. Although large, our sample had, on average, higher household incomes and more educated parents than the general Canadian population. Similarly, given that our sample was selected as preschoolers, and girls with ASD tend to be under-identified at this age, our sample had a smaller proportion of girls than expected in ASD (Ofner et al., 2018). Attrition rates at each time point (6% at T1, 15% at T2, 20% at T3, and 32% at T4), although well within expectations for longitudinal studies of children (van Lieshout et al., 2016), introduce additional concerns regarding generalizability. Furthermore, feeding problems and most predictors and covariates in this study were assessed via parental report. Although parents’ perceptions of feeding problems and other difficulties are important, shared method variance is a limitation. In future research, addition of objective measures (e.g., daily food diaries) would strengthen the study of feeding problems and their associations. Finally, the ongoing Pathways in ASD study that provided our study data did not include a specific validated measure of sensory sensitivities. We attempted to create a measure post hoc from the ASD symptom data available to us, but this measure showed poor reliability and was unfit for inclusion in our analyses. Previous literature has shown a relationship between sensory sensitivity and feeding problems in ASD, without controlling for potential covariates (e.g., Postorino et al., 2015; Suarez et al., 2014). Therefore, it will be important for future studies to examine this relationship using valid measures of sensory sensitivity in the context of other predictors or covariates (e.g., externalizing behavior).
Conclusions
In sum, in our study of preschoolers with ASD, the majority of children showed levels of feeding problems that either started and remained low or declined until they entered school. Therefore, when assessing feeding problems in ASD, it is important to consider them against the backdrop of typical development. That is, it may be appropriate to reassure parents that most children outgrow feeding problems with age. Children with very high levels of feeding problems and co-occurring externalizing behavior may warrant more concern, as they appear to follow a more persistent trajectory. Furthermore, similar to typically developing children, it appears that feeding problems in children with ASD may be part of a wider regulatory problem (i.e., externalizing/internalizing behavior, sleep disturbances, etc.), rather than ASD-specific symptoms. Our results suggest that interventions targeting feeding problems should be considered in the broader context of behavioral or self-regulatory difficulties.
Supplementary Data
Supplementary data can be found at: https://academic.oup.com/jpepsy.
Supplementary Material
Acknowledgments
The authors thank all the children and families who have participated in the Pathways in ASD study. We are also grateful to the current and past members of the study team, and to Charlotte Waddell for her thoughtful feedback on the manuscript.
Funding
This study was supported by the Canadian Institutes of Health Research, Autism Speaks, Government of British Columbia, Alberta Innovates Health Solutions, Kids Brain Health Network (formerly NeuroDevNet), the Sinneave Family Foundation, and the Mayberry Family.
S.P. was supported through the Social Sciences and Humanities Research Council by a Joseph-Armand Bombardier Doctoral Scholarship. I.M.S. was supported by the Joan and Jack Craig Chair in Autism Research. T.B. and S.G. were supported by the Hamilton Health Sciences Early Career Award. P.S. was supported by the Patsy and Jamie Anderson Chair in Child and Youth Mental Health. L.Z. was supported by the Stollery Children’s Hospital Foundation Chair in Autism. T.V. was supported by the Canada Research Chair in Children’s Mental Health and Violence Prevention.
Conflicts of interest: L.Z. is an advisory committee member on a Roche-funded study.
References
- Achenbach, T. M., & Rescorla, L. A. (2000). Manual for ASEBA preschool forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families.
- Allen, S. L., Smith, I. M., Duku, E., Vaillancourt, T., Szatmari, P., Bryson, S., . . . Georgiades, S. (2015). Behavioral Pediatrics Feeding Assessment Scale in young children with autism spectrum disorder: Psychometrics and associations with child and parent variables. Journal of Pediatric Psychology, 60, 581–590. doi:10.1093/jpepsy/jsv006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th edn). Washington, DC: Author. [Google Scholar]
- Bandini L. G., Anderson S. E., Curtin C., Cermak S., Evans E. W., Scampini R., Must A. (2010). Food selectivity in children with autism spectrum disorders and typically developing children. The Journal of Pediatrics, 157, 259–264. doi:10.1016/j.jpeds.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandini L. G., Curtin C., Phillips S., Anderson S. E., Maslin M., Must A. (2017). Changes in food selectivity in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 439–446. doi:10.1007/s10803-016-2963-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighley J. S., Matson J. L., Rieske R. D., Adams H. L. (2013). Food selectivity in children with and without autism spectrum disorder: Investigation of diagnosis and age. Developmental Disabilities, 34, 3497–3503. doi:10.1016/j.ridd.2013.07.026 [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B, 57, 289–300. [Google Scholar]
- Bodfish J. W., Symons F. J., Parker D. E., Lewis M. H. (2000). Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders, 30, 237–243. doi:10.1023/A:1005596502855 [DOI] [PubMed] [Google Scholar]
- Cano S. C., Tiemeier H., Van Hoeken D., Tharner A., Jaddoe V. V., Hofman A., Hoek H. W. (2015). Trajectories of picky eating during childhood: A general population study. International Journal of Eating Disorders, 48, 570–579. doi:10.1002/eat.22384 [DOI] [PubMed] [Google Scholar]
- Crist W., McDonnell P., Beck M., Gillespie C. T., Barrett P., Mathews J. (1994). Behavior at mealtimes and the young child with cystic fibrosis. Journal of Developmental and Behavioral Pediatrics, 15, 157–161. doi:10.1097/00004703-199406000-00001 [Google Scholar]
- Crist W., Napier-Phillips A. (2001). Mealtime behaviors of young children: A comparison of normative and clinical data. Journal of Developmental and Behavioral Pediatrics, 22, 279–286. doi:10.1097/00004703-200110000-00001 [DOI] [PubMed] [Google Scholar]
- Davis A. M., Canter K. S., Stough C. O., Gillette M. D., Patton S. (2014). Measurement of mealtime behaviors in rural overweight children: An exploratory factor analysis of the Behavioral Pediatrics Feeding Assessment Scale. Journal of Pediatric Psychology, 39, 332–339. doi:10.1093/jpepsy/jst089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey, E. E., Smith, I. M., Flanagan, H. E., Duku, E., Lawrence, M. A., Szatmari, P., . . . Bennett, T. (2018). Psychometric properties of the Merrill-Palmer-Revised Scales of Development in preschool children with autism spectrum disorder. Assessment, doi:10.1177/851073191118818754 [DOI] [PubMed] [Google Scholar]
- Dietz, C., Swinkels, S.H., Buitelaar, J.K., van Daalen, E., & van Engeland, H. (2007). Stability and change of IQ scores in preschool children diagnosed with autistic spectrum disorder. European Child & Adolescent Psychiatry, 16, 405–410. doi: 10.1007/s00787-007-0614-3 [DOI] [PubMed] [Google Scholar]
- Dovey T. M., Jordan C., Aldridge V. K., Martin C. I. (2013). Screening for feeding disorders. Creating critical values using the behavioural pediatrics feeding assessment scale. Appetite, 69, 108–113. doi:10.1016/j.appet.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Goodlin-Jones B. L., Sitnick S. L., Tang K., Liu J., Anders T. F. (2008). The Children's Sleep Habits Questionnaire in toddlers and preschool children. Journal of Developmental and Behavioral Pediatrics, 29, 82–88. doi:10.1097/DBP.0b013e318163c39a [DOI] [PubMed] [Google Scholar]
- Gotham K., Pickles A., Lord C. (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 693–705. doi:10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P., Oakland T. (2000). (ABAS-II) Adaptive behaviour assessment system. (2nd edn). Livonia, MN: Pearson Assessments. [Google Scholar]
- Honomichl R. D., Goodlin-Jones B. L., Burnham M., Gaylor E., Anders T. F. (2002). Sleep patterns of children with pervasive developmental disorders. Journal of Autism and Developmental Disorders, 32, 553–561. doi:10.1023/A:1021254914276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. R., Turner K., Stewart P. A., Schmidt B., Shui A., Macklin E., Hyman S. L. (2014). Relationships between feeding problems, behavioral characteristics and nutritional quality in children with ASD. Journal of Autism and Developmental Disorders, 44, 2175–2184. doi:10.1007/s10803-014-2095-9 [DOI] [PubMed] [Google Scholar]
- Kass R. E., Raftery A. E. (1995). Bayes factors. Journal of the American Statistical Association, 90, 773–795. [Google Scholar]
- Kozlowski A. M., Matson J. L., Belva B., Rieske R. (2012). Feeding and sleep difficulties in toddlers with autism spectrum disorders. Research in Autism Spectrum Disorders, 6, 385–390. doi:10.1016/j.rasd.2011.06.012 [Google Scholar]
- Kuschner E. S., Eisenberg I. W., Orionzi B., Simmons W. K., Kenworthy L., Martin A., Wallace G. L. (2015). A preliminary study of self-reported food selectivity in adolescents and young adults with autism spectrum disorder. Research in Autism Spectrum Disorders, 15, 53–59. doi:10.1016/j.rasd.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, K. L., & Aman, M. G. (2007). The Repetitive Behavior Scale-Revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37, 855–866. doi:10.1007/s10803-006-0213-z [DOI] [PubMed] [Google Scholar]
- Ledford J. R., Gast D. L. (2006). Feeding problems in children with autism spectrum disorders: A review. Focus on Autism and Other Developmental Disabilities, 21, 153–166. doi:10.1177/10883576060210030401 [Google Scholar]
- Liu K., Liu J., Xiong X., Yang T., Hou N., Liang X., …Li T. (2016). Correlation between nutrition and symptoms: Nutritional survey of children with autism spectrum disorder in Chongqing, China. Nutrients, 14, E294. doi:10.3390/nu8050294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C., Risi, S., Lambrecht, L., Cook, E. H.Jr, Leventhal, B. L., DiLavore, P. C., . . . Rutter, M. (2000). The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. doi:10.1023/A:100559240194 [PubMed] [Google Scholar]
- Lucyshyn J. M., Fossett B., Bakeman R., Cheremshynski C., Miller L., Lohrmann S., Irvin L. K. (2015). Transforming parent-child interaction in family routines: Longitudinal analysis with families of children with developmental disabilities. Journal of Child and Family Studies, 24, 3526–3541. doi:10.1007/s10826-015-0154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion A., Leader G. (2013). Comorbidity in autism spectrum disorder: A literature review. Research in Autism Spectrum Disorders, 7, 1595–1616. doi:10.1016/j.rasd.2013.09.00 [Google Scholar]
- McDonald C. A., Thomeer M. L., Lopata C., Fox J. D., Donnelly J. P., Tang V., Rodgers J. D. (2015). VABS-II ratings and predictors of adaptive behavior in children with HFASD. Journal of Developmental and Physical Disabilities, 27, 235–247. doi:10.1007/s10882-014-9411-3 [Google Scholar]
- Mirenda, P., Smith, I. M., Vaillancourt, T., Georgiades, S., Duku, E., Szatmari, P., . . . Zwaigenbaum, L. (2010). Validating the Repetitive Behavior Scale-Revised in young children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 40, 1521–1530. doi:10.1007/s10803-010-1012-0 [DOI] [PubMed] [Google Scholar]
- Moffitt T. E., Arseneault L., Belsky D., Dickson N., Hancox R. J., Harrington H., Caspi A. (2011). A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences, 108, 2693–2698. doi:10.1073/pnas.1010076108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskens J. B., Velders F. P., Staal W. G. (2017). Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: A systematic review. European Child & Adolescent Psychiatry, 26, 1093–1103. doi:10.1007/s00787-017-1020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D. S. (1999). Analyzing developmental trajectories: A semi-parametric, group-based approach. Psychological Methods, 4, 139–157. [DOI] [PubMed] [Google Scholar]
- Ofner M., Coles A., Decou M. L., Do M. T., Bienek A., Snider J., Ugnat A.-M. (2018). Autism spectrum disorder among children and youth in Canada 2018: A report of the national autism spectrum disorder surveillance system. Ottawa, ON: Public Health Agency of Canada; Retrieved from https://www.canada.ca/en/public-health/services/publications/diseases-conditions/autism-spectrum- disorder-children-youth-canada-2018.html [Google Scholar]
- Owens J. A., Spirito A., McGuinn M. (2000). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep: Journal of Sleep Research & Sleep Medicine, 23, 1–9. doi:10.1093/sleep/23.8.1d [PubMed] [Google Scholar]
- Pandolfi V., Magyar C. I., Dill C. A. (2009). Confirmatory factor analysis of the Child Behavior Checklist 1.5–5 in a sample of children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 986–995. doi:10.1007/s10803-009-0716-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton S. R., Dolan L. M., Powers S. W. (2006). Parent report of mealtime behaviors in young children with type 1 diabetes mellitus: Implications for better assessment of dietary adherence problems in the clinic. Journal of Developmental and Behavioral Pediatrics, 27, 202–208. doi:10.1097/00004703-200606000-00004 [DOI] [PubMed] [Google Scholar]
- Postorino V., Sanges V., Giovagnoli G., Fatta L. M., De Peppo L., Armando M., Mazzone L. (2015). Clinical differences in children with autism spectrum disorder with and without food selectivity. Appetite, 92, 126–132. doi:10.1016/j.appet.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Rivard, M., Terroux, A., Parent-Boursier, C., & Mercier C. (2014). Determinants of stress in parents of children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 44, 1609–1620. doi: 10.1007/s10803-013-2028-z [DOI] [PubMed] [Google Scholar]
- Rogers L. G., Magill-Evans J., Rempel G. R. (2012). Mothers' challenges in feeding their children with autism spectrum disorder—Managing more than just picky eating. Journal of Developmental and Physical Disabilities, 24, 19–33. doi:10.1007/s10882-011-9252-2 [Google Scholar]
- Roid G., Sampers J. (2004). Merrill-Palmer-Revised Scales of Development. Wood Dale, IL: Stoelting Co. [Google Scholar]
- Rutter, M., Le Couteur, A., & Lord, C. (2003). ADI-R Autism Diagnostic Interview Revised. Manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Sharp W. G., Berry R. C., McCracken C., Nuhu N. N., Marvel E., Saulnier C. A., Jaquess D. L. (2013). Feeding problems and nutrient intake in children with autism spectrum disorders: A meta-analysis and comprehensive review of the literature. Journal of Autism and Developmental Disorders, 43, 2159–2173. doi:10.1007/s10803-013-1771-5 [DOI] [PubMed] [Google Scholar]
- Sparrow, S. S., Cicchetti, D. V., & Balla, D. A. (2005). Vineland Adaptive Behaviour Scales: Second edition (Vineland II), Survey interview form/caregiver rating form. Livonia, MN: Pearson Assessments. [Google Scholar]
- Suarez M. A., Atchison B. J., Lagerwey M. (2014). Phenomenological examination of the mealtime experience for mothers of children with autism and food selectivity. American Journal of Occupational Therapy, 68, 102–107. doi:10.5014/ajot.2014.008748 [DOI] [PubMed] [Google Scholar]
- Suarez M. A., Nelson N. W., Curtis A. B. (2014). Longitudinal follow-up of factors associated with food selectivity in children with autism spectrum disorders. Autism, 18, 924–932. doi:10.1177/1362361313499457 [DOI] [PubMed] [Google Scholar]
- Szatmari P., Georgiades S., Duku E., Bennett T. A., Bryson S., Fombonne E., Thompson A. (2015). Developmental trajectories of symptom severity and adaptive functioning in an inception cohort of preschool children with autism spectrum disorder. JAMA Psychiatry, 72, 276–283. doi:10.1001/jamapsychiatry.2014.2463. [DOI] [PubMed] [Google Scholar]
- van Lieshout M., Luman M., Twisk J. W. R., van Ewijk H., Groenman A. P., Thissen A. J. A. M., Oosterlaan J. (2016). A 6-year follow-up of a large European cohort of children with attention deficit/hyperactivity disorder-combined subtype: Outcomes in late adolescence and young adulthood. European Child and Adolescent Psychiatry, 25, 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Gibbons B. G., Schreck K. A. (2005). Comparing selective eaters with and without developmental disabilities. Journal of Developmental and Physical Disabilities, 17, 299–309. doi:10.1007/s10882-005-4387-7 [Google Scholar]
- Winsper C., Wolke D. (2014). Infant and toddler crying, sleeping and feeding problems and trajectories of dysregulated behaviour across childhood. Journal of Abnormal Child Psychology, 42, 831–843. doi:10.1007/s10802-013-9813-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.