Fig 3. SweD and SweC are Type II membrane proteins.
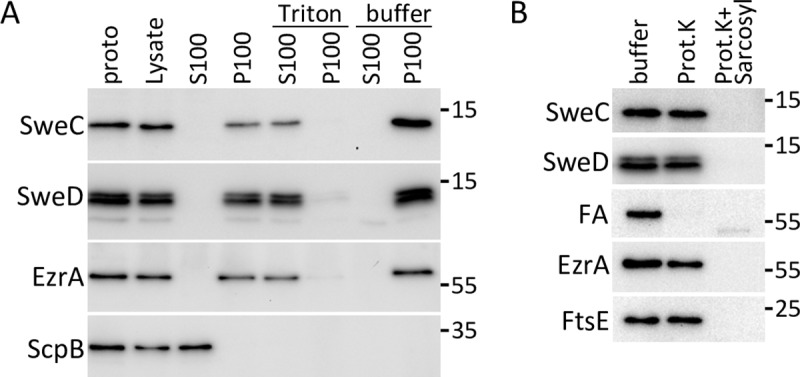
(A) Fractionation of SweD and SweC analyzed by immunoblot. Lysates from exponentially growing wild-type cells were subject to centrifugation to separate soluble (S100) and membrane-associated (P100) proteins. The membrane fraction was incubated with buffer or buffer supplemented with TritonX-100 and the solubilized proteins (S100) were separated from insoluble material (P100) by a second round of centrifugation. Equivalent amounts of each fraction including the input protoplasts (proto) were separated by SDS-PAGE and analyzed by immunoblot. The membrane protein EzrA and cytoplasmic protein ScpB served as membrane and cytoplasmic controls. (B) Protease accessibility analysis of SweD and SweC analyzed by immunoblot. Protoplasts of strain BDR776 were treated with buffer or buffer supplemented with Proteinase K in the absence or presence of sodium-lauroyl-sarcosinate (Sarcosyl). Reactions were resolved by SDS-PAGE and analyzed by immunoblot. SweD and SweC were inaccessible to cleavage by Proteinase K. Degradation of the extracellular domain of SpoIVFA (FA) served as a protease accessible control. The membrane protein EzrA and the cytoplasmic protein FtsE served as protease inaccessible controls. The immunoblots shown are from one of three independent experiments. Molecular weight markers (in kDa) are indicated.
