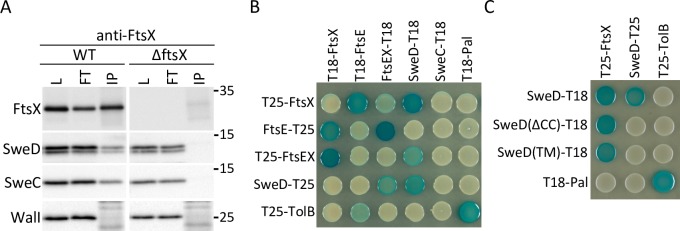
Fig 7. SweD and SweC reside in a complex with FtsX.
(A) Immunoblot analysis of co-immunoprecipitation assays. Digitonin-solubilized membrane fractions derived from exponentially growing wild-type (WT) (PY79) and ΔftsX (BJM72) mutant cells were incubated with anti-FtsX antisera and precipitated with Protein A Sepharose. The detergent-solubilized fraction prior to immunoprecipitation (load; L), the supernatants after immunoprecipitation (flow through; FT), and the immunoprecipitates (IP) were subjected to immunoblot analysis probing for FtsX, SweD, SweC and a control membrane protein WalI. Molecular weight markers (in kDa) are indicated. Immunoblots are representative of one of two independent experiments. Since the anti-FtsX polyclonal antibodies were not covalently coupled to the Protein A Sepharose the heavy and light chains are present in the IP fractions. The bands and smears detected in the FtsX and WalI immunoblots result from the secondary antibodies recognizing light chains that migrate heterogeneously at ~25 kDa. (B) The bacterial adenylate cyclase two-hybrid (BACTH) assay detects an interaction between SweD and FtsX. The BTH101 E. coli reporter strain containing plasmids with the indicated proteins fused to the T18 or T25 domains of the Bordetella adenylate cyclase were spotted on LB(X-gal) indicator plates. Interactions can be detected between FtsE and FtsX, SweD and FtsX, and SweD and itself. T18 and T25 fusions to E. coli TolB and Pal were used as positive and negative controls. (C) Analysis of SweD truncation variants. Interactions can be detected between FtsX and SweD lacking its putative coiled-coil domain (SweDΔCC) and lacking its entire intracellular domain [SweD(TM)]. No interaction was detected between full-length SweD and either of these SweD variants. The BACTH assays were performed in triplicate and photographs of representative agar plates are shown.