Figure 3.
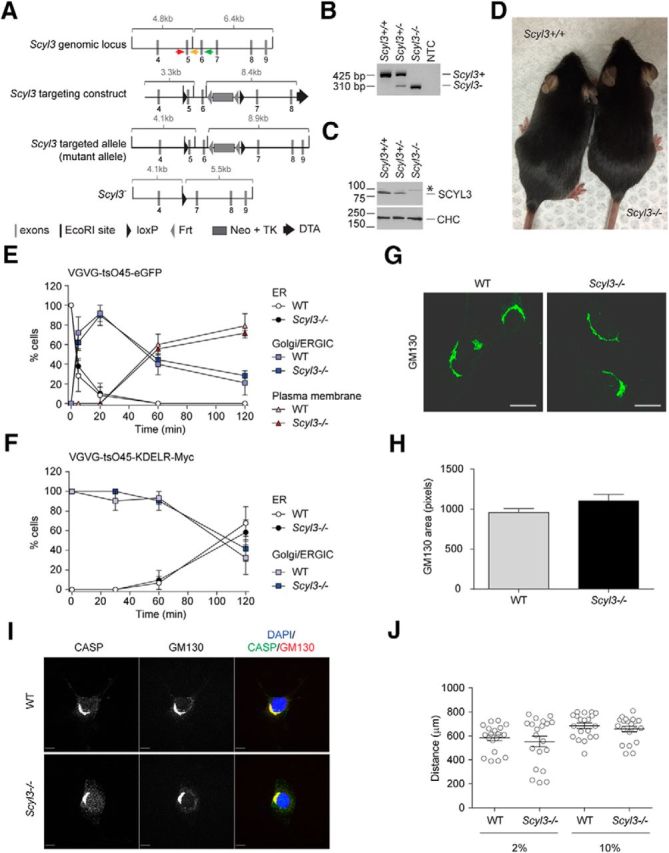
Scyl3 is dispensable for embryonic development and postnatal life, COPI function, Golgi size and morphology, CASP subcellular localization, and cell migration. A, Schematic representation of the Scyl3 locus (introns 3–9), targeting vector, and predicted Scyl3 mutant and null alleles. The Scyl3 gene is located on chromosome 1 and contains 14 exons. Only the region containing exons 4–9 is illustrated. Cre-mediated recombination of the Scyl3 locus to generate the null allele was performed in ES cells. The splicing of exon 4 in exons 7, 8, 9, or 10 causes frame shifts and premature STOP codons. Gray bars, Exons; black bars, EcoRI sites; black triangles, loxP; gray triangles, Frt sites; large gray box, neo-TK cassette; black arrow, diphtheria toxin cassette; red arrow, genotyping primer S3F01; orange arrow, genotyping primer S3R02; green arrow, genotyping primer S3R51. B, PCR-based genotyping of Scyl3+/+, Scyl3+/−, and Scyl3−/− mice. Genomic DNA from Scyl3+/+, Scyl3+/−, and Scyl3−/− mice was amplified by PCR, using primers S3F01, S3R51, and S3R02. Bands of 521 bp and 310 bp correspond to the WT (Scyl3+) and null allele (Scyl3−), respectively. NTC, no template control. C, SCYL3 expression in WT (Scyl3+/+), Scyl3+/−, and Scyl3−/− MEFs. Protein lysates were prepared from exponentially growing MEFs, resolved by SDS-PAGE, and analyzed by Western blot, using antibodies against SCYL3 or clathrin heavy chain (CHC) as loading control. D, Photograph of WT and Scyl−/− mice. Scyl3−/− mice were viable, fertile, and showed no overt abnormalities. E, VSVG-tsO45-eGFP forward movement in WT and Scyl3−/− MEFs. MEFs were transfected with VSVG-tsO45-eGFP. Then, 24 h after transfection, cells were incubated at 40°C for 12 h and then transferred to 32°C for various time points (0, 5, 20, 60, and 120 min), fixed and stained with GFP antibody, and imaged with a fluorescent microscope. The number of cells showing predominant ER, Golgi/ERGIC, or plasma membrane staining was determined and expressed as percentage of total number of cells analyzed for each independently derived cell line (WT, n = 20–25 cells per time point from 3 cell lines; Scyl3−/−, n = 20–25 cells per time point from 3 cell lines). F, VSVG-tsO45-KDELR-Myc retrograde movement in WT and Scyl3−/− MEFs. MEFs were transfected with VSVG-tsO45-KDELR-Myc and incubated at 32°C. Cells were then transferred to the nonpermissive temperature (40°C) for various time points (0, 30, 60, and 120 min) and fixed and stained with an anti-Myc antibody. The number of cells showing predominant ER or Golgi/ERGIC staining was determined and expressed as percentage of total number of cells analyzed for each independently derived cell line (WT, n = 20–25 cells per time point from 3 cell lines; Scyl3−/−, n = 20–25 cells per time point from 3 cell lines). G, H, Golgi size and morphology in WT and Scyl3-deficieint MEFs. G, Exponentially growing WT and Scyl3−/− MEFs were fixed and stained for CASP and GM130 and imaged by confocal microscopy. H, GM130-positive area (pixels) was determined in WT (n = 162 cells from 3 independently derived cell lines) and Scyl−/− MEFs (n = 142 cells from 3 independently derived cell lines). Data are expressed as the mean ± SEM. No significant differences in size or overall morphology were found between WT and Scyl3−/− MEFs. I, Absence of SCYL3 does not affect CASP localization to the Golgi apparatus. Confocal microscopy of exponentially growing WT and Scyl3−/− MEFs, using antibodies against CASP and GM130. Blue, DAPI staining; green, CASP; red, GM130. J, Migration of WT and Scyl3-deficient MEFs. The distance traveled by each cell line (in quintuplicate) over 24 h was measured. Data are expressed as the mean ± SEM of 15 measurements from three independently derived WT and Scyl3−/− MEFs cell lines. Scale bars, 10 μm
