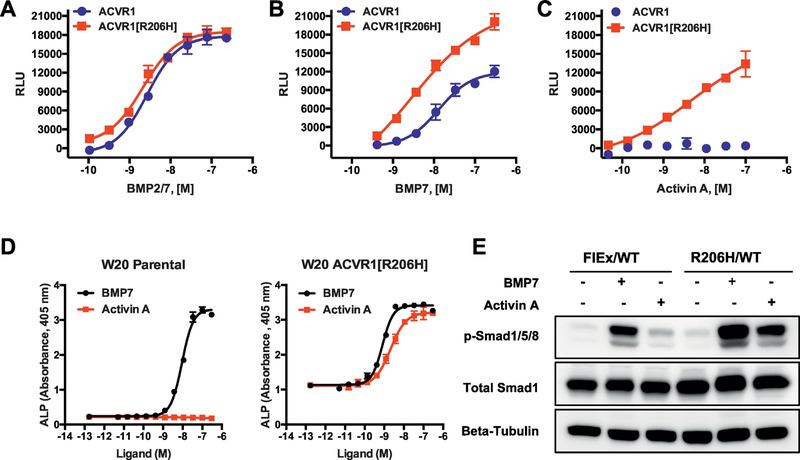
Fig. 3.
Activin A activates ACVR1[R206H] signals through Smad1/5/8 in human and mouse cells, and hence acts much like a BMP. (A, B, C) The responsiveness of ACVR1 or ACVR1[R206H] to different BMP family ligands was tested in HEK293 cells overexpressing either ACVR1 or ACVR1[R206H] and the Smad1/5/8 reporter BRE-luciferase [1]. (A) ACVR1[R206H] and ACVR1 respond equally to some BMPs, including BMP2/7. (B) ACVR1[R206H] displays an enhanced response to some ligands, e.g. BMP7. (C) ACVR1[R206H] responds to Activin A just like it does to a BMP, whereas ACVR1 does not recognize Activin A as an agonistic ligand. (D) Identical results were obtained in the mouse bone marrow stromal cell line W20, expressing either endogenous ACVR1 or overexpressing ACVR1[R206H] and using alkaline phosphatase (ALP) as a readout for activation of Smad1/5/8 signaling. (E) The same pattern is observed in a non-overexpressing system, i.e. mouse embryonic stem (ES) cells, in which the R206H variant has been knocked in (as described in Fig. 1A). Mouse ES cell line Acvr1[R206H]FlEx/+; Gt(ROSA26)SorCreERT2/+ (FlEx/WT) and its post-Cre counterpart, ES line Acvr1[R206H]/+; Gt(ROSA26)SorCreERT2/+ (R206H/WT) were treated with 6 nM BMP7 or 6 nM Activin A for 1 h prior to protein lysate collection. pSmad1/5/8 was measured by Western blotting using beta-tubulin as a loading control. Mirroring results obtained in HEK293 cells and W20 cells, R206H/WT ES cells recognize Activin A as an agonistic ligand, whereas FlEx/WT cells do not. In contrast, both lines respond to BMP7. A more detailed presentation of the responsiveness of ACVR1[R206H] to BMP family ligands can be found in Hatsell, Idone et al. [1] and Dey et al. [3]. The responsiveness of other FOP-causing variants of ACVR1 to Activin A and other BMP family ligands has been examined in detail by Hino et al. [2]. The data presented here was generated as described [1].