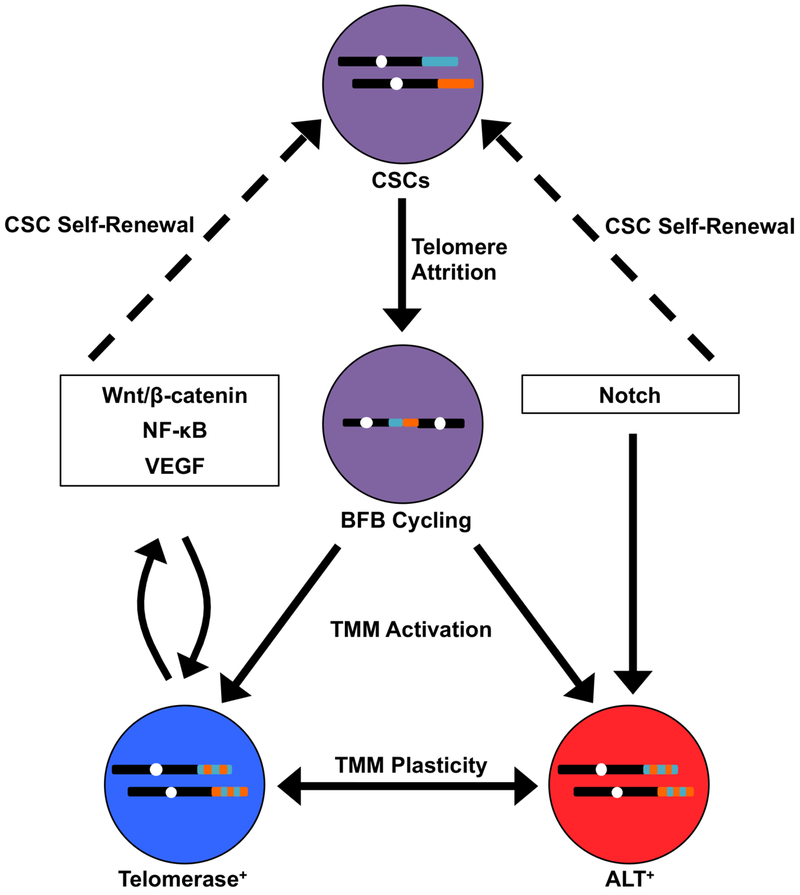
Figure 1. Telomere-centric Model of Breast CSC Biogenesis and Metastatic Evolution.
Cancer stem cells (CSCs; purple) harboring telomeres of a given length (shown for two different chromosomes in teal and orange) undergo telomere attrition as a by-product of self-renewal. This ultimately yields critically short telomeres that are temporarily repaired by chromosome end-to-end fusions, resulting in breakage-fusion-bridge (BFB) cycling (represented by dicentric chromosome). BFB cycling or chromoanagenesis (not shown) cause widespread chromosomal instability (represented by dual-colored telomeres) and the acquisition of new genetic features, including those that are advantageous for metastasis. At the same time, telomere maintenance mechanisms (TMMs) are activated in these new clonal populations, which are defined in part by their reliance on telomerase (blue) or ALT (red). In addition, TMMs exhibit a degree of plasticity, such that TMM identity may interconvert between telomerase and ALT. TMM selection is influenced by signaling pathways that simultaneously promote CSC propagation (dashed arrows). In turn, telomere maintenance proteins directly regulate these signaling pathways, establishing reciprocal feedback loops that coordinate TMM activation and CSC maintenance.