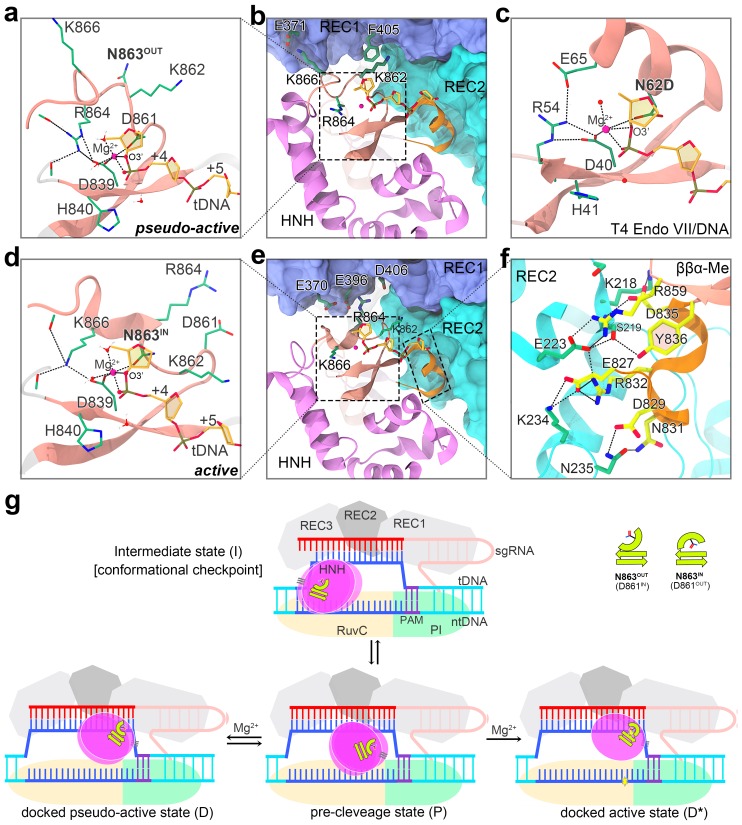
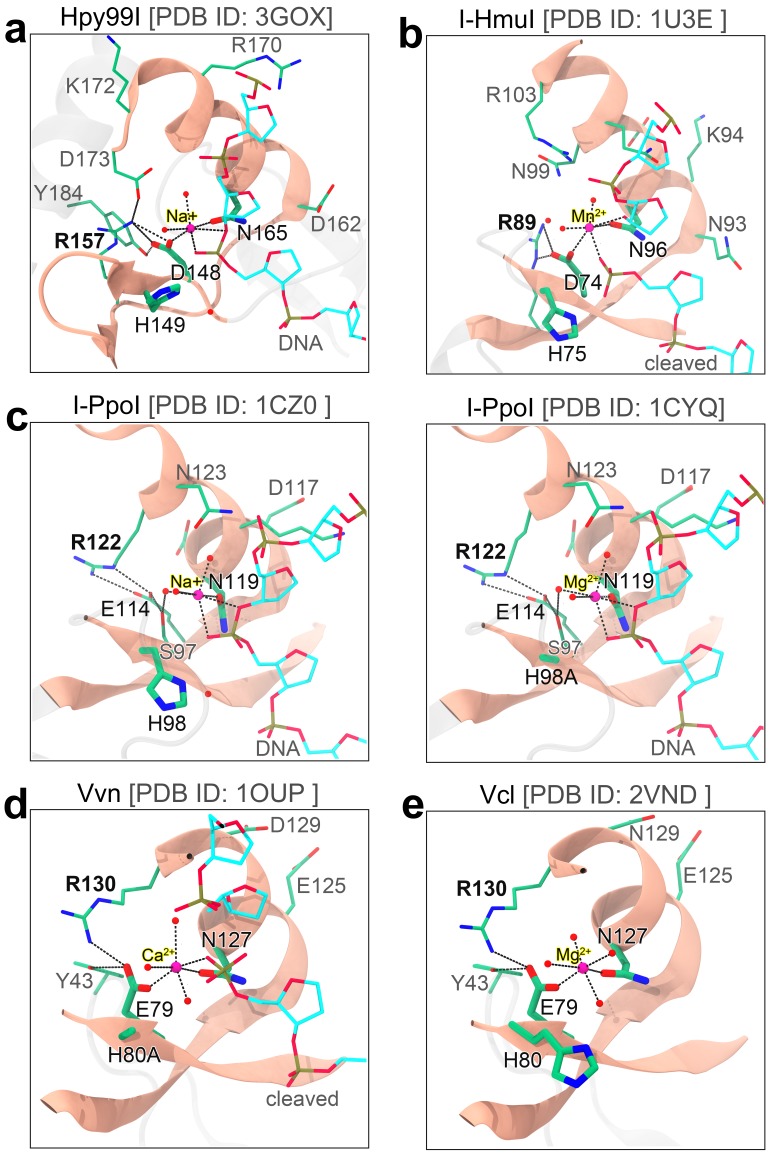
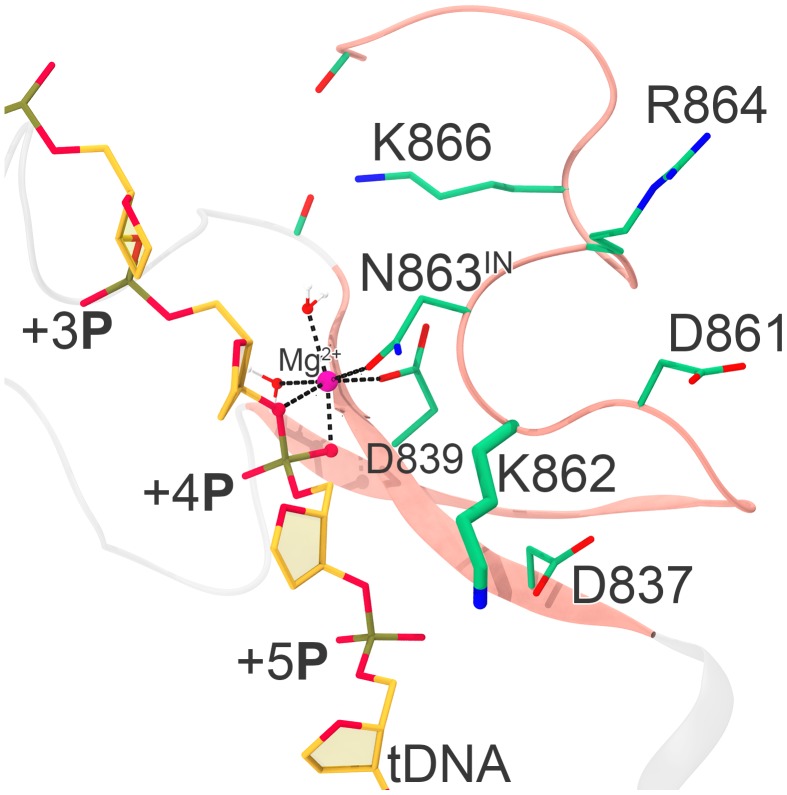
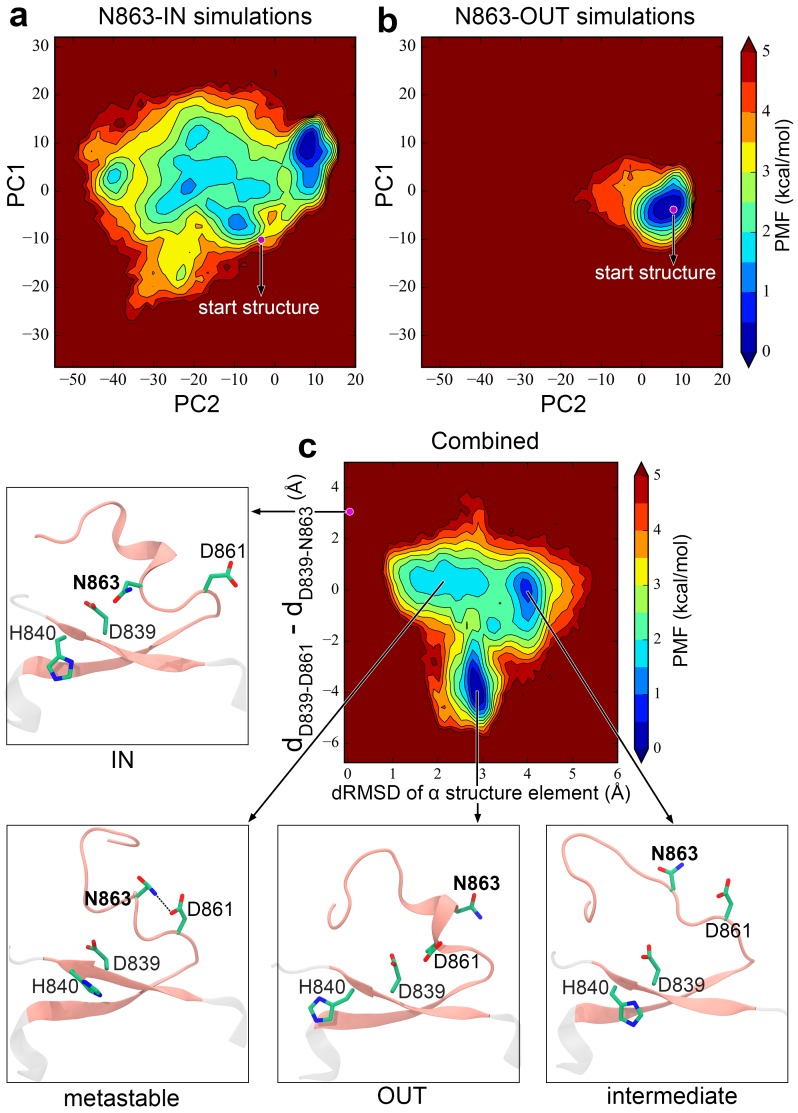
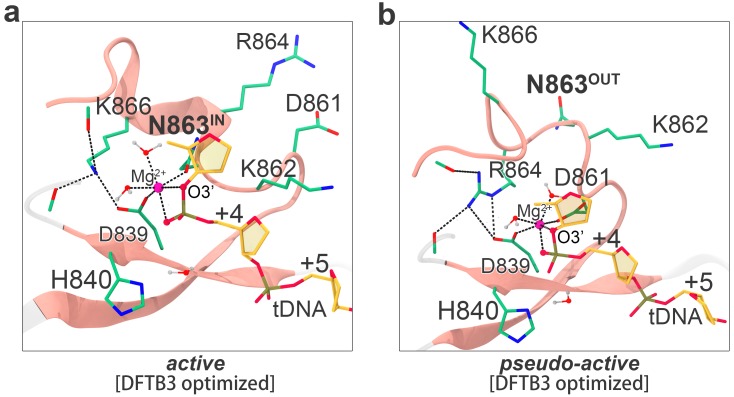
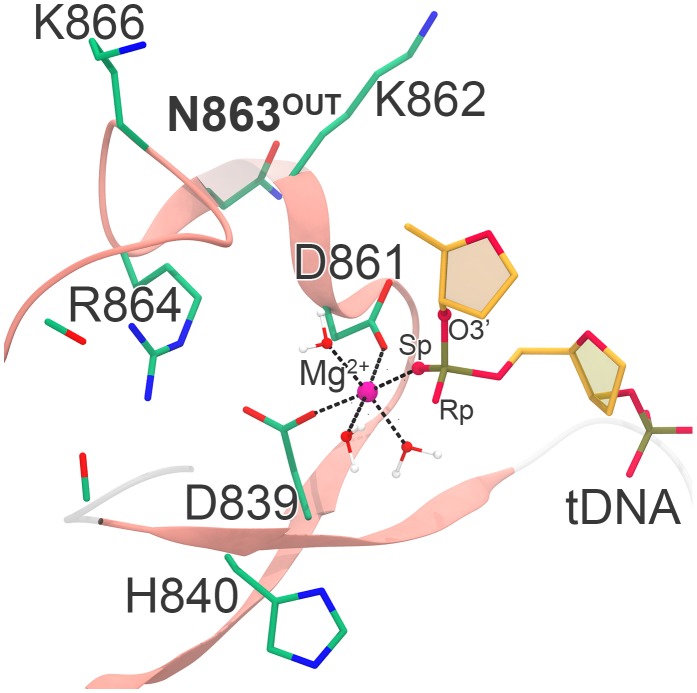
Figure 2. Comparison of the active and pseudoactive Cas9-nucleic acid complex structures and proposed mechanism for DNA cleavage activation of Cas9.
(a–b) Zoomed-in view (a) and zoomed-out view (b) of the HNH domain docked onto the tDNA and REC lobe in the optimized pseudoactive state. (c) Close-up view of the catalytic configuration of the T4 Endo VII (N62D) ββα-Me motif complexed with a DNA substrate. (d–f) Zoomed-in views (d, f) and zoomed-out view (e) of the HNH domain docked onto the tDNA and REC lobe in the catalytically active state. (g) Schematic diagram of the proposed mechanism underlying Cas9 HNH domain conformational activation.