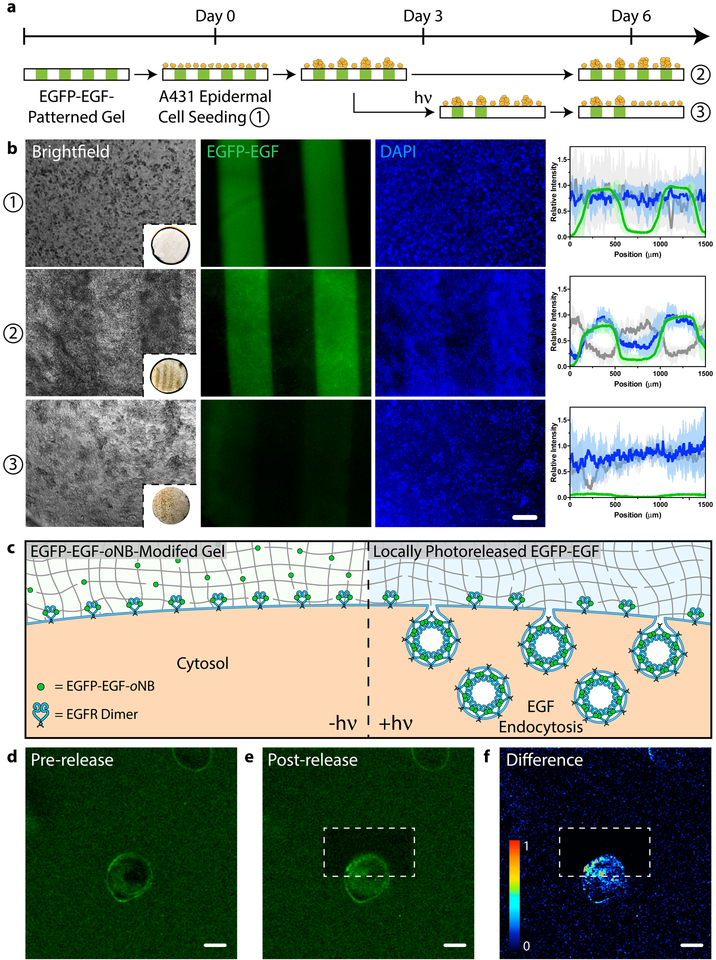
Figure 6 |. Modulating cell fate with a photoreleasable sortagged fluorophore-growth factor chimeric protein.
a. Patterned population-level control of A431 cell proliferation is achieved through stimulation with gel-immobilized EGFP-EGF-oNB-N3 in 2D culture. Protein photoremoval promotes dynamic cell redistribution. Key analytical timepoints are noted on the experimental timeline with circled numbers. b. Brightfield and fluorescent images highlight cell response (nuclei in blue) to patterned EGFP-EGF-oNB-N3 (green) at various experimental endpoints. Relative intensity profiles are given for EGFP (green), nuclei (blue), and optical transmission (grey) across gels perpendicular to photopatterned lines, with average values (dark lines) and standard deviations (light error bars) corresponding to data from three biological replicates (n = 3). Brightfield insets depict full hydrogel (~0.5 cm in diameter). Images and analysis in condition ③ correspond to the gel half whose protein was photoreleased on Day 3. c. Encapsulated cells bind but cannot internalize hydrogel-tethered EGFP-EGF-oNB-N3. Photoliberation of the soluble protein promotes canonical EGFR activation and associated membrane endocytosis. d-e. EGFP-EGF protein release is confined to gel subvolumes bisecting a single A431 cell in 3D culture. Endosome formation is visible in <5 minutes and concentrated in the regions of light exposure. Fluorescent images correspond to timepoints (d) immediately preceding and (e) 5 minutes after protein photorelease within a single gel. f. Difference calculations between images pre- and post-release highlight local EGFP-EGF internalization. Scale bar = 200 μm in b and 10 μm in d-f.