Abstract
Background:
Mailed outreach promoting colorectal cancer (CRC) screening with a stool blood test kit may increase participation, but magnitude and consistency of benefit of this intervention strategy is uncertain.
Aim:
Conduct a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing mailed outreach offering stool tests to usual care, clinic-based screening offers on CRC screening uptake in the United States.
Methods:
We performed a systematic literature search of 5 databases for RCTs of mailed outreach January 1980 through June 2017. Primary outcome was screening completion, summarized using random-effects meta-analysis as pooled differences in proportion completing screening and relative risk of achieving screening compared to control. Subgroup analyses by test type offered--fecal immunochemical test (FIT) or guaiac fecal occult blood test (gFOBT)--, presence of telephone reminders, and presence of predominant underserved/minority population within study were performed. Quality of evidence was evaluated using the GRADE framework.
Results:
7 RCTs which enrolled 12,501 subjects were included (n=5,703 assigned mailed outreach and n=6,798 usual care). Mailed outreach resulted in a 28% absolute (95%CI: 25–30%; I2=47%), and a 2.8-fold relative (RR 2.65, 95%CI: 2.03–3.45; I2=92%) increase in screening completion compared to usual care, with a number needed to invite of 3.6. Similar outcomes were observed across subgroups. Overall body of evidence was moderate quality.
Conclusions:
Mailed outreach offering a gFOBT or FIT is associated with a large and consistent increase in CRC screening completion and should be considered for more widespread implementation for improving screening rates nationwide.
Keywords: Colorectal cancer, cancer screening, meta-analyses, systematic review
INTRODUCTION
Colorectal cancer (CRC) is the 2nd leading cause of cancer deaths in the United States.[1, 2] Screening can prevent mortality and morbidity, but is underutilized. The most recent National Health Interview Survey (NHIS) found that 62% of the US population is up-to-date, a proportion significantly lower than the National Colorectal Cancer Roundtable’s goal of 80% by 2018. Further, examination of data from the last three NHIS surveys from 2011, 2013, and 2015 suggest that screening rates are plateauing, ranging from 60–62%.[3] Evidence-based strategies are required to optimize screening rates, and deliver the promise of CRC screening and prevention to the population.
The Community Guide to Preventive Services (“Community Guide”) has recommended use of multicomponent interventions to promote CRC screening. This is defined as using interventions from at least 2 out of the following 3 domains: 1) Interventions to increase community demand, such as client reminders, client incentives, small media, mass media, group education, one-on-one education; 2) Interventions to increase community access, such as reducing structural barriers, reducing client out-of-pocket costs; and 3) Interventions to increase provider delivery of screening services, such as provider assessment and feedback, incentives, and/or reminders.[4] Mailed outreach offering stool based tests for CRC screening, such as guaiac fecal occult blood testing (gFOBT) or fecal immunochemical testing (FIT), qualifies as multicomponent as mailed outreach usually includes elements from two of the necessary domains: 1) increasing community demand via client reminders and written educational materials, and 2) increasing community access by reducing structural barriers through the delivery of mailed interventions (usually including a free stool test) which eliminates the need for patients to physically go to a medical facility to get the test. More specifically, mailed outreach usually consists of identifying individuals not up-to-date with screening within a health system, mailing invitations to complete screening with an enclosed stool testing kit, and often telephone or written reminders to complete screening. Within health systems, mailed outreach has been shown in multiple randomized trials to significantly increase screening rates. Importantly, many of these trials have been done in settings serving low income, uninsured, and/or minority populations – groups that have historically low rates of screening.
Despite promising results, mailed outreach has not been widely implemented, and has not been formally endorsed by entities such as the National Colorectal Cancer Roundtable. Lack of uptake may be because of varying outcomes, study populations, and type of tests offered (FIT or guaiac FOBT) across studies. We postulate that establishing the magnitude and consistency of benefit of mailed outreach across study populations and test types might help facilitate endorsement and widespread implementation of this strategy for increasing screening rates. Accordingly, our aim was to conduct a systematic review and meta-analysis comparing the impact of mailed outreach on CRC screening compared to usual care, opportunistic offers for screening. We used Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria to appraise the quality of the evidence.
METHODS
Approach
Our overarching goal was to identify randomized controlled trials comparing impact of mailed outreach offering stool-based tests to usual care, opportunistic offers for increasing CRC screening.
Selection Criteria:
We focused our review on randomized controlled trials only; any observational studies were excluded from our review. The primary population within these trials were patients not upto-date with screening. Studies were included if the primary intervention was mailed outreach offering a stool-based screening test, such as FOBT, FIT, or multi-target stool DNA, and the control group was usual care screening, defined as office visit-based opportunistic screening offers. The primary study outcome was the proportion of patients who completed CRC screening on follow-up. Studies were excluded if they featured patients with a higher risk for colorectal cancer (i.e., inflammatory bowel disease), focused on interventions for improving colonoscopy follow-up after abnormal stool tests, did not include a usual care control group, or yielded incomplete data.
Search Strategy:
Electronic searches were performed in MEDLINE/PubMed, EMBASE, CINAHL, Cochrane Central Register of Controlled Trials, and Google Scholar (using the first 200 results) for eligible studies from January 1980 to June 29, 2017. The search strategy included controlled vocabulary terms and keyword terms for each of the four concepts: colorectal cancer, screening or detection, health promotion methods, and type of trial and were not limited by language. A complete description of the search strategies is provided in the Supplementary Materials under Search Strategy. The search was updated for PubMed through July 2018 and yielded no additional papers meeting inclusion criteria.
Study Selection:
Title and abstract review were conducted by a single reviewer by applying preset inclusion/exclusion criteria (MJ). Manuscripts were reviewed by two reviewers (MJ, AA), with discrepancies being sent to the senior author (SG) for resolution. Only published studies with full manuscripts were included.
Data Extraction:
Data extraction was conducted by two reviewers (MJ, AA), with discrepancies being resolved via discussion. The following data were extracted from each included study: 1) study characteristics, including author, year published; 2) Study population characteristics, including whether the study included substantial representation of underserved populations such as uninsured and/or minorities, age, proportion female; 3) Interventions utilized, including the test offered (gFOBT, FIT, or multi-target stool DNA), number of stool samples requested, telephone reminders, and any additional interventions ; 4) Outcomes, including the number of participants who completed screening in each group.
Risk of Bias Assessment
Risk of bias assessment in these trials was performed using the Cochrane Risk of Bias tool. Studies were deemed to be at high, low, or unclear risk of bias based on adequacy of sequence generation, allocation concealment, blinding, method of addressing incomplete data, selective reporting, and other biases.
Data Synthesis and Statistical Analysis
Primary outcome of interest was completion of CRC screening within nine months to one year of intervention. Additionally, we planned a priori to examine results stratified by test type used (FIT or gFOBT), presence of telephone reminders as part of the intervention, and whether the study population focused on underserved/minority populations.
We used the random effects model described by DerSimonian and Laird to estimate pooled risk difference (magnitude of difference between active intervention and control) and relative risk (of achieving outcome as compared to control) and 95% confidence intervals (CI).[5] We assessed heterogeneity between study-specific estimates by measuring the percentage of variation across studies due to heterogeneity (I2), and used cut-offs of <30%, 30%−59%, 60%−75% and >75% to suggest low, moderate, substantial and considerable heterogeneity, respectively. Between-study sources of heterogeneity were assessed in subgroup analyses defined above using meta-regression. A p-value for differences between subgroups of <0.10 was considered statistically significant. Due to small number of studies, a reliable assessment of publication bias could not be estimated. All analysis was performed using Comprehensive Meta-Analysis (CMA) version 3 (Biostat, Englewood, NJ) and Stata version 13 (StataCorp, College Station, TX). We estimated number needed to treat (NNT) from the summary estimates for the primary outcome, using the median control group risk of studies included in the meta-analysis. Specifically, we defined NNT follows: NNT = 1/ (pooled median intervention effect – median control group effect). This systematic review is registered at PROSPERO, protocol registration number CRD42017070542.
GRADE Quality of Evidence
We followed the GRADE approach to appraise the confidence in estimates derived from meta-analysis of efficacy outcomes. In this approach, direct evidence from RCTs starts at high confidence and can be rated down based on risk of bias, indirectness, imprecision, inconsistency (or heterogeneity) and/or publication bias, to levels of moderate, low and, very low confidence. Quality of evidence was assessed by two authors (MJ, KS), with discrepant ratings resolved by discussion.
RESULTS
Search Results
Our literature search yielded a total of 1,088 candidate studies for inclusion. After title review, 725 studies were selected for abstract review. Abstract review yielded 28 studies for detailed manuscript review. [6–27] Of these, 6 were found to be duplicates and 15 did not meet inclusion/exclusion criteria[6, 7, 23–27, 8–12, 18, 21, 22], leaving 7 for final inclusion[13–17, 19, 20] (see Supplementary Materials, Figure A for search flow, and Table A for exclusion details for papers that underwent manuscript review).
Study characteristic
The 7 selected RCTs were published between 2004 and 2016, and in total included 12,501 individuals who were randomly assigned to mailed outreach (n=5,703) or usual care (n=6,798). FIT was utilized for 3 of 7 studies and gFOBT for 4 of 7 studies. Sample size ranged from 119 to 5,491. Proportion female ranged from 54% to 73%. Underserved populations such as those with low income, or who were uninsured or minorities were the focus of 6 out of 7 studies. See Table 1 for detailed characteristics of included studies.
Table 1.
Detailed characteristics of included randomized trials comparing mailed outreach with usual care for colorectal cancer screening
| Study | Test Type | Telephone Reminder | Majority Underserved Population | # Allocated, Usual Care | # Allocated, Mailed Outreach | Mean Age, Usual Care (SD) | Mean Age, Mailed Outreach (SD) | # Female, Usual Care (%) | # Female Mailed Outreach (%) | Screening Completion Usual Care (%) | Screening Completion, Mailed Outreach (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Singal, 2016(18) | FIT | Yes | Yes | 1199 | 2400 | 56 (4) | 56 (4) | 744 (62%) | 1474(61%) | 355 (30%) | 1410 (59%) |
| Gupta, 2013(19) | FIT | Yes | Yes | 3898 | 1593 | 59 (3) | 59 (3) | 2517 (65%) | 993 (62%) | 471 (12%) | 648 (41%) |
| Jean-Jacques, 2012**(20) | gFOBT | Yes | Yes | 98 | 104 | 60 (8) | 60 (7) | 58 (59%) | 67 (64%) | 15 (15%) | 40 (38%) |
| Coronado, 2011(21) | gFOBT | No | Yes | 167 | 168 | Unknown | Unknown | 88 (53%) | 89 (53%) | 4 (2%) | 43 (26%) |
| Goldberg, 2004(22) | gFOBT | No | Yes | 60 | 59 | 64 | 64 | 46 (77%) | 42 (71%) | 3 (5%) | 24 (41%) |
| Goldman, 2015(23) | FIT | Yes | Yes | 210 | 210 | 57 (6) | 58 (6) | 138 (66%) | 139 (66%) | 47 (22%) | 84 (40%) |
| Green, 2013(24) | gFOBT | No | No | 1166 | 1169 | Unknown | Unknown | 653 (56%) | 629 (54%) | 301 (26%) | 659 (56%) |
primary outcome data was for screening at 4 month follow up, but since data for 12 month follow up were provided, study was included and 12 month outcome data were abstracted for analysis; FIT, fecal immunochemical test; gFOBT, guaiac fecal occult blood test
Impact of mailed outreach on screening completion
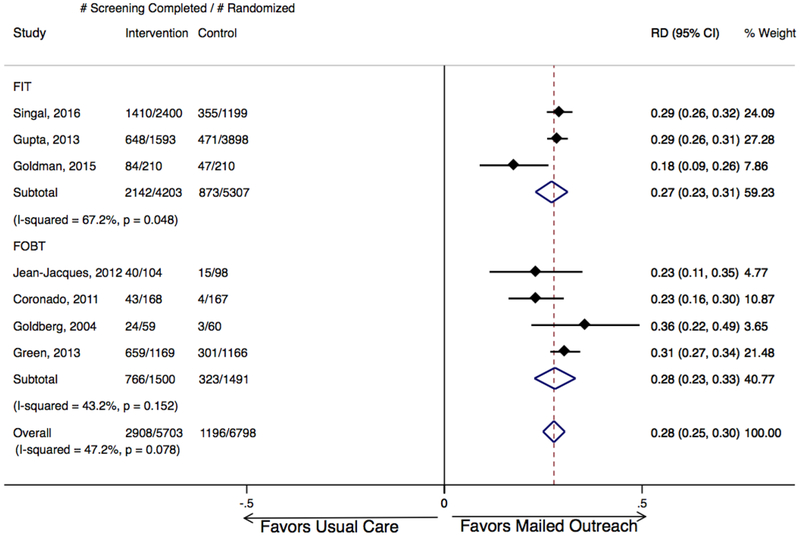
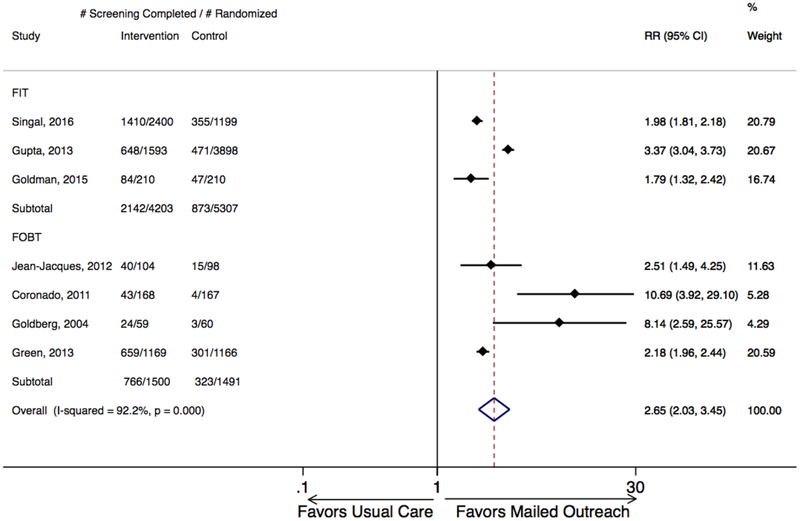
Mailed outreach offering stool tests was associated with a statistically significant absolute improvement in CRC screening in each of the 7 included studies, ranging from 18% to 36% (Figure 1). Across studies, proportion completing screening ranged from 20- to 9% overall, from 2 to 30% among usual care groups, and from 26 to 59% among mailed outreach groups. Meta-analysis demonstrated mailed outreach was associated with a 28% absolute increase in screening compared to usual care, office visit-based opportunistic offers for screening with moderate heterogeneity (95% CI: 25–30%; I2=47%; Figure 1), and 2.65-fold increased chance of screening completion (RR 2.65, 95% CI: 2.03–3.45; I2=92%; Figure 2). Based on these results, number needed to invite to achieve one additional patient up-to-date with screening was estimated to be 3.6.
Figure 1. Absolute increase in CRC screening completion for mailed outreach vs. usual care, stratified by test type (FIT or gFOBT).
Mailed outreach was associated with a pooled absolute increase in screening completion of 28% for all studies, 27% for studies employing FIT, and 28% for studies employing gFOBT. Weights are from random effects analysis. CRC, colorectal cancer; FIT, fecal immunochemical test; gFOBT, guaiac fecal occult blood test; RD, relative difference.
Figure 2. Relative increase in CRC screening completion for mailed outreach vs. usual care, stratified by test type (FIT or gFOBT).
Mailed outreach was associated with a pooled 2.6-fold relative increase in screening completion for all studies, a 1.79-fold relative increase for studies employing FIT, and a 2.18-fold relative increase for studies employing gFOBT. Weights are from random effects analysis. CRC, colorectal cancer; FIT, fecal immunochemical test; gFOBT, guaiac fecal occult blood test; RD, relative difference.
Subgroup Analyses
Analyses stratified by stool test type found the 3 studies offering FIT were associated with a 27% absolute increase in screening (95% CI: 23–31%; I2=67%), while the 4 studies offering gFOBT were associated with a 28% increase in screening (95% CI: 23–33%; I2=43%; Figure 1). Similar magnitude of screening increases, and p-value for significant difference of stool test groups of 0.61 suggested that stool test type had no major impact on efficacy of mailed outreach.
Analyses stratified by presence of telephone reminders found the 4 studies including telephone reminders were associated with a similar increase in screening as the 3 studies without telephone reminders: absolute increase 27% with reminders (95% CI: 23–31; I2=56%) vs. 29% without reminders (95% CI: 23–35; I2=54%); p-value for significant difference in risk = 0.49.
Analyses stratified by focus on underserved and/or minority populations found similar impact of mailed outreach within settings providing care to populations at high risk for screening non-completion. Specifically, the pooled absolute increase in screening observed for the 6 studies focusing on underserved and/or minority populations was 27% (95% CI: 23–30%; I2=49%), while the increase in screening for the 1 study not focusing on underserved/minority populations was 31% (95% CI: 27–34; p-value for difference in risk differences = 0.40).
Quality Assessment:
The included studies were judged to be at low (n=3) and moderate (n=4) risk of bias (Supplementary Materials, Table B). For studies judged to be at moderate risk of bias, the most common limitation was a lack of both participant and provider (double) blinding, but we determined that this was unlikely to significantly influence the results of the studies, as the screening rates were likely not affected. Taken together, because the 7 studies included are at low/moderate risk of bias, we conclude that the strength of evidence to support effectiveness of mailed outreach for promoting CRC screening completion based on this meta-analysis is moderate (Supplementary Materials, Table C).
DISCUSSION
In this systematic review and meta-analysis of RCTs comparing mailed outreach promoting CRC screening with included stool blood kits to usual care, clinic-based opportunistic offers for screening, we observed that mailed outreach was associated with a large pooled 28% absolute increase in screening, with moderate confidence in estimates. Overall, this corresponds to a number needed to invite of 3.6. The analysis included data contributed by 7 randomized trials representing over 12,501 patients and showed consistent results. Specifically, a similar absolute increase was seen across subgroup analyses stratifying by test offered (FIT or gFOBT) and presence of telephone reminders. Remarkably, 6 of 7 studies focused on underserved populations, highlighting potential for mailed outreach to improve screening among populations with traditionally low screening participation. Taken together, the results suggest that mailed outreach offering stool blood tests increases screening completion with a large effect size, compared to usual care.
Our results, together with prior work by others, indicate that mailed outreach should undergo more widespread implementation for increasing screening. The 28% pooled absolute increase in screening associated with mailed outreach versus usual care compares very favorably with other interventions for increasing screening. For example, the observed increase for mailed outreach compared to usual care is superior to that observed for mailed colonoscopy offers (10.2%) and visit-based FOBT offers (7.7%)[4]. Furthermore, the 28% screening increase from mailed outreach significantly exceeds that interventions such as offering FIT or FOBT at time of seasonal Flu vaccination campaigns (15%)[29], patient reminders (5–15%[30], 10%[31]), and patient education (17%)[7]. Mailed outreach was comparable to screening increases from eliminating structural barriers (15–42%)[30] and one-on-one interactions (15–42%)[30]. The 20 to 49% range of screening completion in response to mailed outreach observed in the US studies included in our review is generally lower than observed in studies from Europe (range 20 to 68%) and Asia (range 21 to 63%) [28]. However, the generally lower ranges may be because usual care, opportunistic screening is not offered at baseline (individuals offered outreach are more likely to be naive to prior screening offers) and because outreach is offered in context of national healthcare systems.
Our results complement other recent systematic reviews of strategies for promoting screening. In a comprehensive systematic review of population health interventions to improve screening studied by randomized trials or observational studies, Issaka et al. also concluded mailed outreach was highly effective relative to other interventions such as offering FIT at time of flu vaccination [32]. Similarly, Davis et al. conducted a systematic review of clinic and community interventions shown by randomized trials and observational studies to increase stool occult blood testing in rural and low-income populations in the United States and concluded multicomponent interventions such as mailed outreach were among the most promising strategies for boosting screening [33]. These comprehensive systematic reviews of randomized and non-randomized studies are complemented by our meta-analysis which quantitatively synthesizes the net effect of CRC screening with mailed outreach vs. usual care as reported by randomized trials. Dougherty conducted a broad systematic review and meta analysis of interventions intended to increase CRC screening rates in the US, and reported that fecal occult blood test outreach, defined as mailed outreach or distributing FITs at time of patient encounters such as flu vaccination, was associated with 2-fold increased likelihood of screening completion [34]. Our meta-analysis is complimentary, but specifically focuses only on mailed outreach, and required a usual care comparator arm for all included studies. Taken together, the preponderance of available evidence suggests that mailed outreach should be more strongly considered as a strategy for increasing screening. Several limitations should be considered when interpreting our results. First, a number of high quality mailed outreach studies were not included based on a priori exclusion criteria such as lack of data on screening 9 to 12 months post randomization [9, 25, 26], including individuals with an increased risk of CRC[32], and lack of a usual care control group defined as office visit-based opportunistic screening offers to patients overdue for CRC screening[12, 27]. Notably, the direction and magnitude of mailed intervention effects in these excluded studies were similar to that observed across included studies. Second, we focused on studies that utilized usual care, opportunistic screening as a control group. We focused on these studies because in the United States, opportunistic screening is the most common approach. Additionally, we focused on stool-based tests, since this is the most common approach to mailed outreach, though some have shown that mailed outreach offering alternate tests such as colonoscopy may also be successful for increasing screening[13, 14]. Strengths of this study include our focus on studies relevant to understanding the incremental benefit of mailed outreach over usual care, a rigorous a priori search strategy, and critical appraisal of quality of evidence as part of our methods.
Overall, our results suggest that mailed outreach offering either gFOBT or FIT is a highly effective, evidence-based strategy for increasing CRC screening, including among underserved populations. Given that we are still short of the 80% by 2018 goal set by the National Colorectal Cancer Roundtable for population screening, mailed outreach should be strongly considered as an additional strategy for increasing screening and be more widely implemented by health systems.
Supplementary Material
ACKNOWLEDGMENTS:
Funding for this project was provided by the National Cancer Institute of the National Institutes of Health under award numbers: U54CA132384, U54CA132379, and 1R37CA222866–01. The project described was also supported by Merit Review Award number 1 I01 HX001574–01A1 from the United States Department of Veterans Affairs Health Services Research & Development Service of the VA Office of Research and Development. The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosures: Siddharth Singh is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23DK117058, and also by the American College of Gastroenterology Junior Faculty Development Award and the Crohn’s and Colitis Foundation Career Development Award.
Human Subjects: Not applicable to systematic review
Informed consent: Not applicable. For this type of study formal consent is not required
Conflicts of interest: The authors have no relevant conflicts of interest to disclose.
Contributor Information
Mark Jager, San Diego State University.
Josh Demb, Division of Gastroenterology, Department of Medicine, University of California San Diego.
Ali Asghar, Department of Medicine, University of California San Diego.
Kevin Selby, Department of Ambulatory Care and Community Medicine, University of Lausanne.
Evelyn Marquez, Kaiser Permanente, Panorama City.
Karen M. Heskett, University of California San Diego Library
Alicea J. Lieberman, Rady School of Management, University of San Diego.
Zhuo Geng, Division of Gastroenterology, Hepatology, and Nutrition, University of Minnesota.
Balambal Bharti, Moores Cancer Center, UC San Diego
Siddharth Singh, Division of Gastroenterology, Division of Biomedical Informatics, University of California San Diego.
Samir Gupta, VA San Diego Healthcare System, Division of Gastroenterology and the Moores Cancer Center, UC San Diego.
REFERENCES
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A (2017) Colorectal Cancer Facts and Figures 2017–2019. Am. Cancer Soc. [DOI] [PubMed] [Google Scholar]
- 2.(2018) SEER*Explorer Application: Colon and Rectum Long-Term Trends in SEER Incidence Rates, 1975–2015. In: Natl. Cancer Inst. Surveillance, Epidemiol. End Results Progr. https://seer.cancer.gov/explorer/application.php?site=20&data_type=1&graph_type=1&compareBy=sex&chk_sex_1=1&chk_race_1=1&chk_race_3=3&chk_race_2=2&chk_age_range_9=9&advopt_precision=1&advopt_display=1&showDataFor=race_1_and_age_range_9. [Google Scholar]
- 3.(2017) Colorectal Cancer Screening: Cancer Trends Progress Report. In: Centers Dis. Control Prev. https://progressreport.cancer.gov/detection/colorectal_cancer. [Google Scholar]
- 4.(2016) Colorectal Cancer Screening: Multicomponent Interventions--Colorectal Cancer. In: Community Guid. https://www.thecommunityguide.org/findings/cancer-screening-multicomponent-interventions-colorectal-cancer. [Google Scholar]
- 5.DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–88. [DOI] [PubMed] [Google Scholar]
- 6.Lairson DR, DiCarlo M, Deshmuk AA, et al. (2014) Cost-effectiveness of a standard intervention versus a navigated intervention on colorectal cancer screening use in primary care. Cancer 120:1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlton ME, Mengeling MA, Halfdanarson TR, Makki NM, Malhotra A, Klutts JS, Levy BT, Kaboli PJ (2014) Evaluation of a home-based colorectal cancer screening intervention in a rural state. J Rural Heal 30:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendren S, Winters P, Humiston S, Idris A, Li SXL, Ford P, Specht R, Marcus S, Mendoza M, Fiscella K (2014) Randomized, controlled trial of a multimodal intervention to improve cancer screening rates in a safety-net primary care practice. J Gen Intern Med 29:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman RM, Steel SR, Yee EFT, Massie L, Schrader RM, Moffett ML, Murata GH (2011) A system-based intervention to improve colorectal cancer screening uptake. Am J Manag Care 17:49–55. [PubMed] [Google Scholar]
- 10.Marquez E, Singh S, Gupta S (2016) Su1032 Mailed Outreach for Promoting Colorectal Cancer Screening: A Systematic Review and Meta-Analysis of Randomized Trials. Gastroenterology 150:S450. [Google Scholar]
- 11.Geng ZZ, Gupta S (2013) Mo1097 Interventions to Increase Colorectal Cancer Screening Among Underserved Populations: A Systematic Review. Gastroenterology 144:S–576. [Google Scholar]
- 12.Church TR, Yeazel MW, Jones RM, Kochevar LK, Watt GD, Mongin SJ, Cordes JE, Engelhard D (2004) A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J Natl Cancer Inst 96:770–80. [DOI] [PubMed] [Google Scholar]
- 13.Singal AG, Gupta S, Tiro JA, et al. (2016) Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer 122:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta S, Halm EA, Rockey DC, et al. (2013) Comparative Effectiveness of Fecal Immunochemical Test Outreach, Colonoscopy Outreach, and Usual Care for Boosting Colorectal Cancer Screening Among the Underserved. JAMA Intern Med 173:1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jean-Jacques M, Kaleba EO, Gatta JL, Gracia G, Ryan ER, Choucair BN (2012) Program to improve colorectal cancer screening in a low-income, racially diverse population: a randomized controlled trial. Ann Fam Med 10:412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R (2011) Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer 117:1745–1754. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg D, Schiff GD, McNutt R, Furumoto-Dawson A, Hammerman M, Hoffman A (2004) Mailings timed to patients’ appointments. Am J Prev Med 26:431–435. [DOI] [PubMed] [Google Scholar]
- 18.Damery S, Smith S, Clements A, Holder R, Nichols L, Draper H, Clifford S, Parker L, Hobbs R, Wilson S (2012) Evaluating the effectiveness of GP endorsement on increasing participation in the NHS Bowel Cancer Screening Programme in England: study protocol for a randomized controlled trial. Trials 13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman SN, Liss DT, Brown T, Lee JY, Buchanan DR, Balsley K, Cesan A, Weil J, Garrity BH, Baker DW (2015) Comparative Effectiveness of Multifaceted Outreach to Initiate Colorectal Cancer Screening in Community Health Centers: A Randomized Controlled Trial. J Gen Intern Med 30:1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green BB, Wang C-Y, Anderson ML, Chubak J, Meenan RT, Vernon SW, Fuller S (2013) An Automated Intervention With Stepped Increases in Support to Increase Uptake of Colorectal Cancer Screening. Ann Intern Med 158:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha TC, Yong SK, Yeoh K-W, Kamberakis K, Yeo RMC, Koh GC-H (2014) The effect of test kit provision, and individual and family education on the uptake rates of fecal occult blood test in an Asian population: a randomized controlled trial. Cancer Causes Control 25:1473–1488. [DOI] [PubMed] [Google Scholar]
- 22.Levy BT, Xu Y, Daly JM, Ely JW (2013) A Randomized Controlled Trial to Improve Colon Cancer Screening in Rural Family Medicine: An Iowa Research Network (IRENE) Study. J Am Board Fam Med 26:486–497. [DOI] [PubMed] [Google Scholar]
- 23.Walsh JME, Salazar R, Terdiman JP, Gildengorin G, Pérez-Stable EJ (2005) Promoting use of colorectal cancer screening tests. J Gen Intern Med 20:1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JK, Groessl EJ, Ganiats TG, Ho SB (2011) Cost-effectiveness of a mailed educational reminder to increase colorectal cancer screening. BMC Gastroenterol 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh JME, Salazar R, Nguyen TT, Kaplan C, Nguyen L, Hwang J, McPhee SJ, Pasick RJ (2010) Healthy Colon, Healthy Life. Am J Prev Med 39:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ (2009) Patient and Physician Reminders to Promote Colorectal Cancer Screening. Arch Intern Med 169:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green BB, Anderson ML, Chubak J, Baldwin LM, Tuzzio L, Catz S, Cole A, Vernon SW (2016) Colorectal Cancer Screening Rates Increased after Exposure to the Patient-Centered Medical Home (PCMH). J Am Board Fam Med 29:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro M, Nicolas A, Ferrandez A, Lanas A (2017) Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol 23:3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potter MB, Ackerson LM, Gomez V, Walsh JME, Green LW, Levin TR, Somkin CP (2013) Effectiveness and reach of the FLU-FIT program in an integrated health care system: a multisite randomized trial. Am J Public Health 103:1128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R (2010) Systematic Review: Enhancing the Use and Quality of Colorectal Cancer Screening. Ann Intern Med 152:668. [DOI] [PubMed] [Google Scholar]
- 31.Sabatino SA, Lawrence B, Elder R, et al. (2012) Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med 43:97–118. [DOI] [PubMed] [Google Scholar]
- 32.Levy AR, Bruen BK, Ku L (2012) Health care reform and women’s insurance coverage for breast and cervical cancer screening. Prev Chronic Dis 9:E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis MM, Freeman M, Shannon J et al. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States - How, what and when? BMC Cancer. 2018;18(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dougherty MK, Brenner AT, Crockett SD et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis JAMA Intern Med. 2018;78(12):1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.