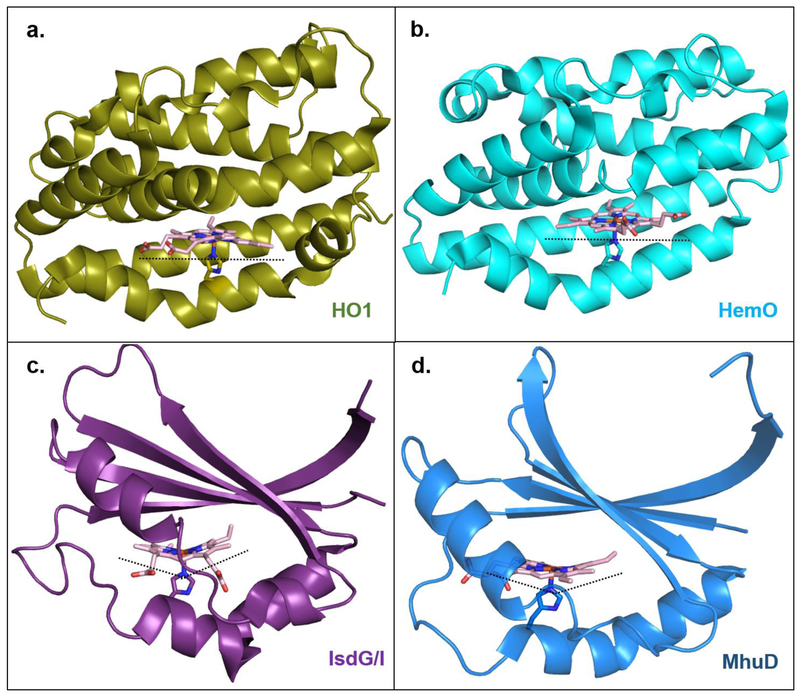
Figure 8. Multiple enzymes, multiple binding modes, multiple ways to degrade heme.
Canonical heme oxygenase enzymes HO1 and HemO ((a); PDBID: 1N45 and (b); PDBID: 1SK7), respectively) bind heme in a planar orientation. HO1 stereospecifically produces α-biliverdin, while HemO produces β- and γ-biliverdin due to the ~100° rotation of the heme ring in this active site relative to HO1. IsdG-like heme oxygenase enzymes IsdG/I and MhuD ((c); PDBID: 2ZDO and (d); PDBID: 4NL5, respectively) “ruffle” the heme porphyrin which results in cleavage of meso-carbons and products that are different than canonical HO’s (see figure 7).The heme ring in MhuD is rotated ~90° relative to the heme in IsdG/I, also contributing to the different bilin products that are produced.