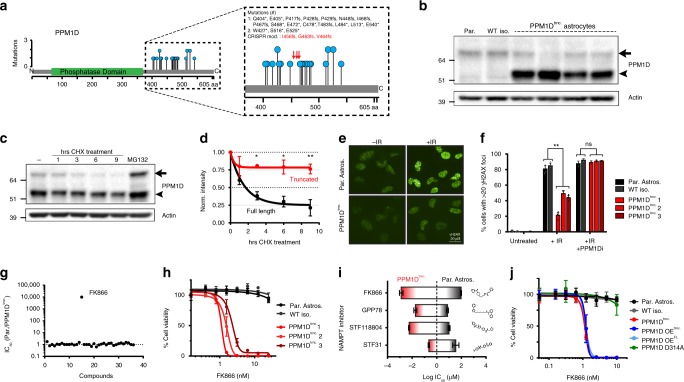
Fig. 1.
PPM1D mutant immortalized human astrocytes are sensitive to NAMPT inhibitors. a Previously identified (refs. 8–10) PPM1D truncation mutations in pediatric HGGs (blue circles). CRISPR-modified mutations in human astrocytes shown in red arrows. b Immunoblot of PPM1D full-length (full arrow) and truncated (arrowhead) protein expression across parental astrocytes (Par.), an isolated wild type astrocyte clone (WT iso.), and four different isolated CRISPR-modified, PPM1D-truncated (PPM1Dtrnc.) astrocytes. c Immunoblot of PPM1D expression post cycloheximide (CHX) and MG132 treatment. d Quantification of the experiment in (c), (n = 3 biologically independent experiments, *p < 0.05, **p < 0.01 by Student’s T test). e Representative images of cellular γH2AX foci, +/− treatment with 10 Gy ionizing radiation (IR). f Quantification of γH2AX foci in untreated, IR-treated, and concurrent IR plus 50 nM PPM1D inhibitor GSK2830371 treatment (PPM1Di); (n = 4 biologically independent samples, **p < 0.001 by Student’s T test). g Calculated IC50 ratios (Parental/PPM1Dtrnc.) for a library of tested small molecule inhibitors. h Viability assessment of wild type (Par. Astros. and WT iso.) and three PPM1Dtrnc. cell lines, 72hrs post FK866 treatment (n = 3 biologically independent samples). i Calculated IC50 values of parental (black highlight) and PPM1Dtrnc. (red highlight) astrocytes for different NAMPT inhibitors; length of bar represents selectivity window of the given drug for PPM1D mutant cells (n = 2 biologically independent experiments). j Viability analysis of cell lines in response to 72 h of FK866 treatment (n = 3 biologically independent samples). All error bars represent standard deviation of the mean