Abstract
Background
Aneurysmal bone cysts (ABC) are benign but locally aggressive lesions. The treatment of ABC has evolved over the years, but curettage with or without local adjuvants still represents the standard. Less invasive methods such as embolization, sclerotherapy or RANKL inhibitors (Denosumab) are also established. The aim of this study was to report and compare the results of a series of patients mainly treated with curettage with and without subsequent phenolization.
Methods
65 patients with the unequivocal diagnosis of primary ABC were treated. 61 of them were located within the bone whereas 4 patients had an ABC of the soft tissues. All patient were treated surgically by means of curettage with or without adjuvants, resection, or with minimally invasive methods such as Polidocanol injections, embolizations or Denosumab treatment. In total 80 procedures had been performed.
Results
Our patients had a mean age of 25.3 ± 16.0 years, ranging from 4 to 74 years. The most common skeletal locations were the pelvis in 23%, the femur in 18%, the tibia in 16% and the spine in 10%. Six lesions were resected and showed no recurrence. 5 patients were treated with polidocanol injections (n = 3) or embolization plus systemic treatment with Denosumab (n = 2). With embolization and Denosumab both patients showed stable disease and required no further treatment. Polidocanol injections resulted in stable disease with no further treatment required in one patient and in subsequent curettage with adjuvant phenolization in two other patients.
In 54 initial curettages 21 were performed with adjuvant phenolization. In this group, 16 lesions healed (76%), 3 showed persistent disease and 2 patients had a local recurrence (9%). Out of 33 patients without phenolization 21 (64%) healed, 3 showed stable persistent disease and 9 (27%) experienced a recurrence. In total we performed 66 curettages, 27 with and 39 without adjuvant phenol treatment. Resolution was achieved in 19 (70%) and 25 (64%) of cases. respectively. Persistent disease was evident in 5 cases each and recurrence in 3 and 9 cases, respectively (n.s.).
Conclusion
Curettage is still the standard of treatment for ABC. Local recurrence does not depend on the use of adjuvant phenol as shown in this and other studies. Minimally invasive methods such as selective embolization and injections of sclerosing agents may result in healing or at least in tolerable persistence of residual lesions but needs repetitive treatments and does not show homogenous results throughout the institutions. Denosumab appears to be an additional option, especially in surgically critical locations such as the spine or the sacrum.
Keywords: Aneurysmal bone cyst, Curettage, Recurrence, Phenol
1. Introduction
Aneurysmal bone cyst (ABC) are benign intraosseous or rarely soft tissue lesions and were first described by Jaffe and Liechtenstein in 1942 [1]. ABC's are considered benign yet locally aggressive lesions with a potential for local recurrence, and they typically appear in the metaphysis of the long bones and in the vertebral column [2], [3]. ABC's are most often seen in children and young adults with no sex predilection. These lesions are lytic, usually eccentrically located and expansive with well-defined margins. There are blood-filled, separated by fibrous septa, with fibroblasts, osteoclast-type giant cells and reactive woven bone [4]. Soft tissue lesions are rare but since 1972 have been described in a number of cases [5].
Aneurysmal bone cysts were originally thought to be reactive in nature, caused by a circulatory abnormality leading to an increased venous pressure and resulting in dilation of the vascular network [6], [7]. Nowadays, the neoplastic nature of aneurysmal bone cyst has been proven since in 1999, Panoutsakopoulos et al. demonstrated a balanced chromosomal translocation t(16;17)(q22;p13) as a cytogenetic abnormality in primary aneurysmal bone cyst [8] involving the USP6 gene, located on chromosome 17p13. After establishing this USP6 translocation as a diagnostic tool, it has been found in approximately 75% of the cases [9]. Thus differentiating primary ABC`s from secondary lesions or other tumors such as teleangiectatic osteosarcoma had become much more easier.
The treatment concepts of ABC have evolved over the years. Resection is not an option in most of the cases leaving intralesional procedures such as curettage as standard of care [10]. Due to local recurrence rates of more than 50%, various adjuvant treatments have been used. Most common are PMMA bone cement, argon beam, phenol, ethanol and cryotherapy [10]. Less invasive methods such as aggressive biopsy (“Curopsy”) [11], selective arterial embolization [12], [13], sclerotherapy with ethibloc or polidocanol [14] and systemic therapy with RANKL inhibitors (Denosumab) [15] have been tried. The aim of this study was to report and compare the results of a series of patients mainly treated by curettage with and without adjuvant phenol treatment and also by less invasive interventions.
2. Material and methods
Between 1982 and 2014, 65 patients with histologically proven primary ABC were treated at our institution. 61 of these ABC were located in bone whereas 4 patients had an ABC of the soft tissues. The medical records and imaging studies of all cases were reviewed after obtaining institutional review board approval and each patient was either contacted by means of a telephone survey or seen in the outpatient clinic. All patient had been treated surgically by curettage with or without adjuvants, resections, or with minimally invasive methods as Polidocanol injections, embolizations or Denosumab therapy. In total, 80 procedures were performed.
2.1. Statistical analysis
For statistical analysis, the recurrence-free survival was calculated according to the Kaplan-Meier method. Significance analysis was performed using the Log-Rank test or the Chi-Square test, respectively. The data analysis software used was MedCalc®.
3. Results
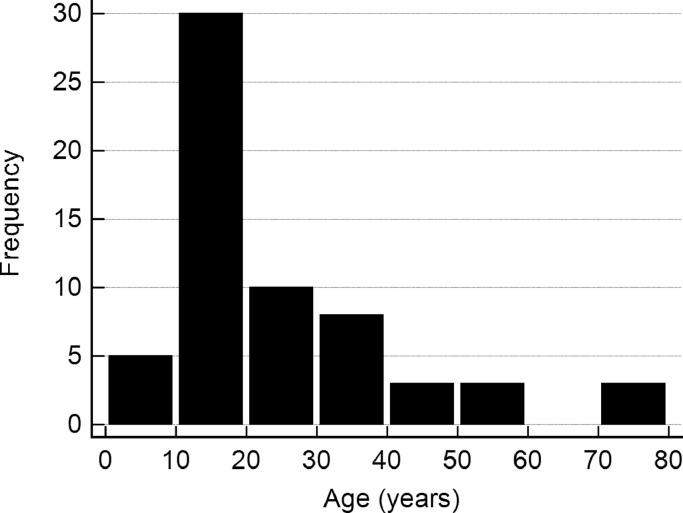
Our patients had a mean age of 25.3 ± 16.0 years at the time of treatment, ranging from 4 to 74 years, most of them were in their second decade of age (Fig. 1). The male to female ratio was 0.94. The most common skeletal locations were the pelvis in 23%, the femur in 18%, the tibia in 16% and the spine in 10% followed by the humerus in 8%, the hand and the foot in 5% each, radius and ulna in 3% each and patella, ribs and fibula in one case each.
Fig. 1.
Age distribution in 65 patients with aneurysmal bone cysts.
The main symptoms that had led to the diagnosis were pain in 89% and local swelling in 15%. As measured on cross-sectional imaging, the mean maximum diameter of the lesions was 5.2 cm ± 4.0 cm, ranging from 1 to 30 cm.
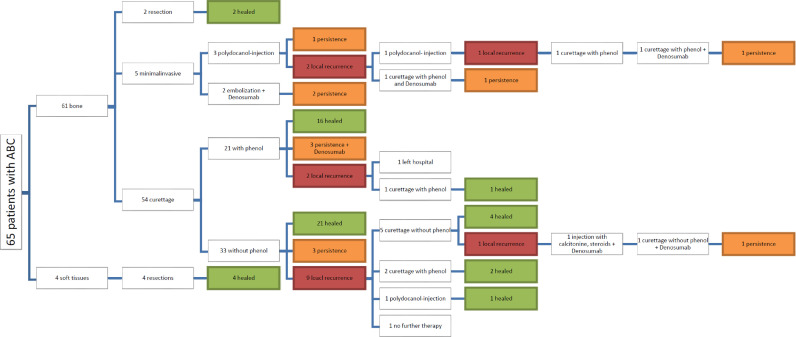
The therapy and outcomes are shown in Fig. 2. Six lesions were resected (4 soft tissue and 2 bone) and showed no recurrence. 5 patients were treated with polidocanol injections (n = 3) or embolization and Denosumab (n = 2). With embolization and Denosumab both patients showed stable disease and need no further treatment. Polidocanol injections caused stable disease with no further treatment in one patient and curettage with adjuvant phenolization led to the same result in two additional patients. Of those, one patient showed stable persistent disease after the procedure, the second needed a second curettage including now also phenol and Denosumab which lead to stable persistent disease.
Fig. 2.
Type of intervention and outcomes in 65 patients with ABC.
Out of 54 primary curettages, 21 were performed with adjuvant phenolization. In this group, 16 lesions healed (76%), 3 showed persistent disease (one with adjuvant Denosumab therapy) and 2 patients had a local recurrence. One of those 2 recurrences had a second curettage with phenol and subsequently healed, the other opted for treatment at another institution with the final outcome unknown.
Out of 33 primary curretages without adjuvant phenolization, 21 (64%) healed, 3 showed stable persistent disease and 9 (27%) had a recurrence. Out of those 9 recurrent cases, 7 had a second curettage which resulted in healing in 6 patients and in persistent disease even after a third curettage and Denosumab treatment in one patient. One recurrence healed after a polidocanol injection and one patient opted for no further therapy.
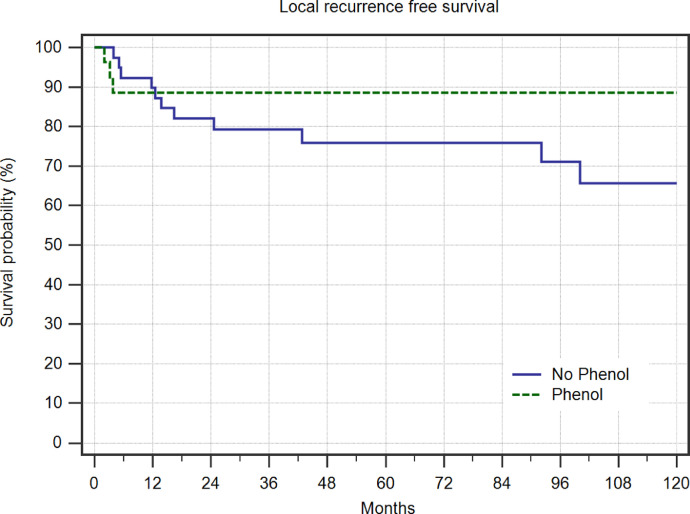
In total, we performed 66 curettages, 27 with and 39 without adjuvant phenolization. Healing was achieved in 19 (70%) and in 25 (64%) cases, respectively. Persistent disease was evident in 5 cases each and recurrence in 3 and 9 cases, respectively (Fig. 3, n.s.). Overall, 80 interventions were performed on 65 patients.
Fig. 3.
Local recurrence free survival in 39 patients treated with curettage alone and 27 patients treated with curettage and adjuvant phenolization.
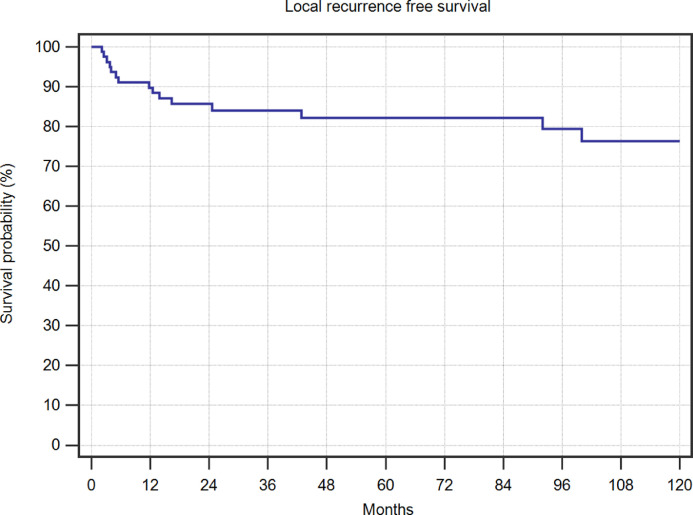
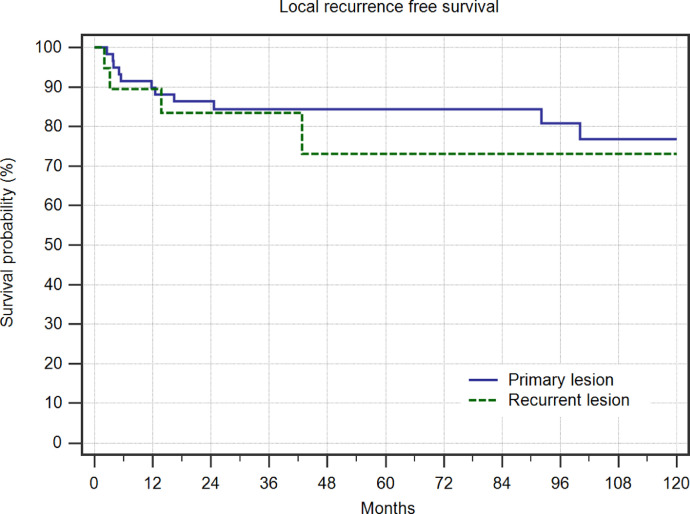
The total mean follow-up was 119 months (median 82 months, range 3.1–408 months). Local recurrence was seen in 15 (19%) patients and with most of them (80%) during the first 2 years after initial treatment, but one patient as late as more than 8 years after the intervention (Fig. 4). In cases where the lesion had already been a recurrent disease, the risk of further recurrence was increased, but this difference did not reach significance in our series (Fig. 5). A comparison of this data to the published series is listed in Table 1.
Fig. 4.
Local recurrence free survival in 80 interventions in patients with ABC.
Fig. 5.
Local recurrence free survival in 60 interventions in patients with primary ABC and 20 patients with recurrent ABC.
Table 1.
A comparison of this data to published series of patients with primary aneurysmal bone cysts.
| Resection |
Curettage |
Polidocanol |
Denosumab |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | SD | PD | CR | SD | PD | CR | SD | PD | CR | SD | PD | |
| This study | 6 (100%) | 0 (0%) | 0 (0%) | 44 (67%) | 10 (15%) | 12 (18%) | 0 (0%) | 1 (33%) | 2 (67%) | 0 (0%) | 2 (100%) | 0 (0%) |
| Basarir et al. [26] | 19 (100%) | 0 (0%) | 0 (0%) | 27 (77%) | 8 (23%) | |||||||
| Brosjö et al. [27] | 37 (97%) | 1 (3%) | ||||||||||
| Dormans et al. [28] | 37 (18%) | 8 (82%) | ||||||||||
| Erol et al. [29] | 5 (100%) | 0 (0%) | 0 (0%) | 52 (93%) | 0 (0%) | |||||||
| Flont et al. [30] | 10 (100%) | 0 (0%) | 0 (0%) | 14 (85%) | 2 (15%) | |||||||
| Gibbs et al. [31] | 6 (100%) | 0 (0%) | 0 (0%) | 30 (88%) | 4 (12%) | |||||||
| Kececi B. et al. [17] | 9 (100%) | 0 (0%) | 0 (0%) | 66 (87%) | 0 (0%) | 10 (13%) | ||||||
| Kurucu et al. [32] | 6 (67%) | 3 (33%) | ||||||||||
| Kurucu et al. [32] | 6 (67%) | 3 (33%) | ||||||||||
| Mankin et al. [33] | 120 (80%) | 30 (20%) | ||||||||||
| Palmerini et al. [15] | 2 (22%) | 7 (78%) | 0 (0%) | |||||||||
| Peeters et al. [34] | 76 (95%) | 4 (5%) | ||||||||||
| Ramirez et al. [35] | 21 (72%) | 8 (28%) | ||||||||||
| Rastogi et al. [36] | 48 (67%) | 22 (30%) | 2 (3%) | |||||||||
| Reddy et al. [11] | 162 (85%) | 28 (15%) | ||||||||||
| Schreuder et al. [34] | 76 (95% | 4 (5%) | ||||||||||
| Schulte et al. [37] | 33 (80%) | 8 (20%) | ||||||||||
| Shooshtarizadeh et al. [38] | 33 (87%) | 0 (0%) | 5 (13%) | |||||||||
| Solooki et al. [39] | 4 100% | 0 (0%) | 0 (0%) | 30 (94%) | 0 (0%) | 2 (6%) | ||||||
| Varshney et al. [23] | 39 (85%) | 0 (0%) | 7 (15%) | 42 (93%) | 0 (0%) | 3 (7%) | ||||||
| Vergel De dios et al. [3] | 17 (100%) | 0 (0%) | 0 (0%) | 107 (79%) | 0 (0%) | 29 (21%) | ||||||
| Wang et al. [21] | 30 (97) | 0 (0%) | 1 (3%) | |||||||||
4. Discussion
ABC is a benign but locally aggressive lesion with no unequivocally defined primary treatment option. En bloc resection is not an option for the majority of patients because of the resulting disability/the required reconstructive surgery on the background of a benign disease. So intralesional curettage with or without bone grafting is still the predominantly used therapy, but carries a risk of local recurrence of about 20% [3]. Phenol, introduced in 19th century medicine as carbolic acid, has long been used for achieving locally aseptic conditions or “sterilization” of remaining tumor cells after intralesional procedures as for example with giant cell tumor (GCT) of bone [16]. In a comparison of curettage with or without adjuvant phenolization in 43 versus 19 patients with ABC, the local recurrence rates were 14% vs. 16%, indicating that this adjuvans has no additional benefit [17]. This is confirmed by our present study. In GCT, the adjuvant effect of phenol is still a subject of discussion. Our own results using the same technique as in ABC showed a trend towards fewer recurrences but without achieving statistical significance [18]. In a recently published report by Nithyananth et al., the minimum time for effective action of phenol against giant cell tumour (GCT) cells was found to be 3 min (67% cell death) with 6 min being required for maximum efficacy (100% cell death) [19]. In our series, we used 50% phenol dissolved in 75% ethanol on swaps, maintained in the curettage cavity for 1 min. So this might have influenced the effect of the substance.
With high speed burring the same conflicting data is evident: no effect at all [17], [20] or only one recurrence in 31 patients [21]. Argon beam coagulation, cryosurgery and cementation have either the risk of osteonecrosis and fracture or leave a biologically inert implant in a meta-epiphyseal location in children or young adults [10].
So over the years, less invasive procedures were propagated. Ethibloc, an alcoholic solution of a fibrogenic and thrombogenic agent proved to be effective with repeated injections but in some cases showed severe side effects [22]. In a randomized study with 94 patients, repeated injections of polidocanol showed better results than curettage alone (93% vs 85% healing) [23]. In our own series of patients presented here, polidocanol either led to local recurrence or to persistent disease.
Repeated arterial embolizations have also been reported with success rates of 80% and higher [12].
One group of investigators also described repeated injections of doxycycline, an antibiotic with some potential to inhibit matrix metalloproteinases and angiogenesis. Shiels et al. reported a healing rate of 94% with this method [24].
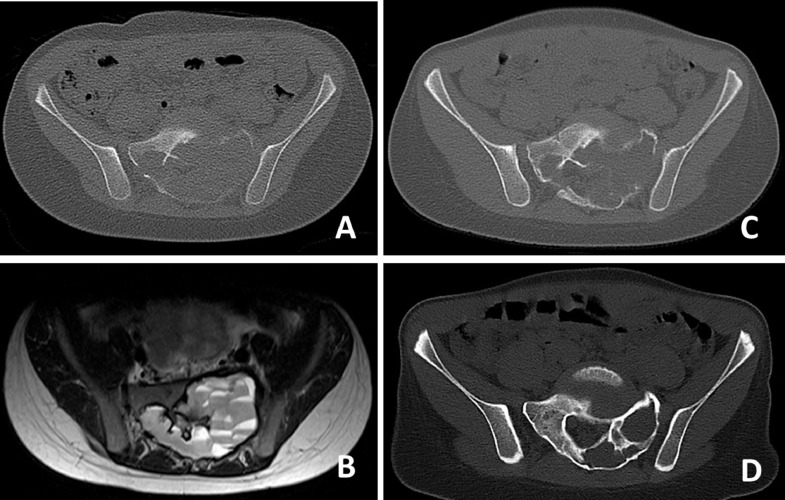
With a high rate of recalcification in GCT of bone, Denosumab, a human monoclonal antibody against the kappa B ligand (RANKL) which promotes osteoclast activation is also being used in ABC. In a recent case series of 9 patients, 5 were still under therapy, 2 patients had to be operated on and 2 patients showed stable disease after one and two years, respectively [15]. Our own experience is quite favorable. Two patients showed stable disease after cessation of Denosumab (Fig. 6), in 3 others, Denosumab was used after curettage. 2 patients showed stable small residual disease, one patient progressed and is again being treated with Denosumab. But we did also observed severe acute hypercalcemia following anti-RANKL withdrawal in children as described in several case reports [25].
Fig. 6.
CT-scan (A) showing massive osteolytic destruction of the sacrum at the levels S1 and S2 caused by ABC. (B) The lesions shows typical fluid- fluid levels on T2-weighted TSE imaging with the patient in supine position, which is a sign of internal bleeding within the cysts. (C) CT-scan two months after initiation of treatment with Denosumab showing initial sclerotic demarcation of the lesion and increased sclerosis. (D) CT-scan after one year of treatment demonstrating stable osseus reconstitution of both sacral ala.
5. Conclusion
Resection of aneurysmal bone cysts results in a local recurrence rate of 0% but is only feasible in very selected cases which is why curettage still represents the standard of treatment. Local recurrence does not depend on the use of adjuvant phenol as shown in this and other studies. Minimally invasive methods such as selective embolization and injections of sclerosing agents or Doxycycline may result in healing or at least in tolerable persistence of residual lesions but require repetitive treatments and have so far not shown homogenous results between different institutions. Denosumab is a recent additional option especially in surgically critical locations such as the spine or the sacrum.
CRediT authorship contribution statement
Ferdinand Grahneis: Data curation, Formal analysis, Investigation, Visualization, Writing - original draft, Writing - review & editing. Alexander Klein: Methodology, Project administration, Writing - original draft, Writing - review & editing. Andrea Baur-Melnyk: Data curation, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Thomas Knösel: Data curation, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Christof Birkenmaier: Methodology, Project administration, Writing - original draft, Writing - review & editing. Volkmar Jansson: Methodology, Project administration, Writing - original draft, Writing - review & editing. Hans Roland Dürr: Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Ethics approval and consent to participate
This study was approved by the ethics committee of the Medical Faculty, University of Munich. Written consent was obtained from all patients included in this study.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study did not have any grants or funding despite the academic setting of the institutions and authors.
Acknowledgements
Not applicable.
References
- 1.Jaffe H.L., Lichtenstein L. Solitary unicameral bone cyst: with emphasis on the roentgen picture, the pathologic appearence and the pathogenesis. Arch. Surg. 1942;44:1004–1025. [Google Scholar]
- 2.Martinez V., Sissons H.A. Aneurysmal bone cyst. A review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 1988;61(11):2291–2304. doi: 10.1002/1097-0142(19880601)61:11<2291::aid-cncr2820611125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Vergel De Dios A.M., Bond J.R., Shives T.C., McLeod R.A., Unni K.K. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69(12):2921–2931. doi: 10.1002/1097-0142(19920615)69:12<2921::aid-cncr2820691210>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Sciot R., Dorfman H., Brys P., Dal Cin P., De Wever I., Fletcher C.D., Jonson K., Mandahl N., Mertens F., Mitelman F., Rosai J., Rydholm A., Samson I., Tallini G., Van den Berghe H., Vanni R., Willen H. Cytogenetic-morphologic correlations in aneurysmal bone cyst, giant cell tumor of bone and combined lesions. A report from the CHAMP study group. Mod. Pathol. 2000;13(11):1206–1210. doi: 10.1038/modpathol.3880224. [DOI] [PubMed] [Google Scholar]
- 5.Pietschmann M.F., Oliveira A.M., Chou M.M., Ihrler S., Niederhagen M., Baur-Melnyk A., Durr H.R. Aneurysmal bone cysts of soft tissue represent true neoplasms: a report of two cases. J. Bone Joint. Surg. Am. 2011;93(9):e45. doi: 10.2106/JBJS.J.00534. [DOI] [PubMed] [Google Scholar]
- 6.Clough J.R., Price C.H. Aneurysmal bone cyst: pathogenesis and long term results of treatment. Clin. Orthop. Relat. Res. 1973;(97):52–63. doi: 10.1097/00003086-197311000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Kransdorf M.J., Sweet D.E. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am. J. Roentgenol. 1995;164(3):573–580. doi: 10.2214/ajr.164.3.7863874. [DOI] [PubMed] [Google Scholar]
- 8.Panoutsakopoulos G., Pandis N., Kyriazoglou I., Gustafson P., Mertens F., Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26(3):265–266. doi: 10.1002/(sici)1098-2264(199911)26:3<265::aid-gcc12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Baumhoer D., Amary F., Flanagan A.M. An update of molecular pathology of bone tumors. Lessons learned from investigating samples by next generation sequencing. Genes Chromosomes Cancer. 2019;58(2):88–99. doi: 10.1002/gcc.22699. [DOI] [PubMed] [Google Scholar]
- 10.Park H.Y., Yang S.K., Sheppard W.L., Hegde V., Zoller S.D., Nelson S.D., Federman N., Bernthal N.M. Current management of aneurysmal bone cysts. Curr. Rev. Musculoskelet. Med. 2016;9(4):435–444. doi: 10.1007/s12178-016-9371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy K.I., Sinnaeve F., Gaston C.L., Grimer R.J., Carter S.R. Aneurysmal bone cysts: do simple treatments work? Clin. Orthop. Relat. Res. 2014;472(6):1901–1910. doi: 10.1007/s11999-014-3513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi G., Mavrogenis A.F., Facchini G., Bartalena T., Rimondi E., Renzulli M., Andreone A., Durante S., Angelini A., Errani C. How effective is embolization with N-2-butyl-cyanoacrylate for aneurysmal bone cysts? Int. Orthop. 2017;41(8):1685–1692. doi: 10.1007/s00264-016-3364-3. [DOI] [PubMed] [Google Scholar]
- 13.Terzi S., Gasbarrini A., Fuiano M., Barbanti Brodano G., Ghermandi R., Bandiera S., Boriani S. Efficacy and safety of selective arterial embolization in the treatment of aneurysmal bone cyst of the mobile Spine: a retrospective observational study. Spine (Phila Pa 1976) 2017;42(15):1130–1138. doi: 10.1097/BRS.0000000000002017. [DOI] [PubMed] [Google Scholar]
- 14.Batisse F., Schmitt A., Vendeuvre T., Herbreteau D., Bonnard C. Aneurysmal bone cyst: a 19-case series managed by percutaneous sclerotherapy. Orthop. Traumatol. Surg. Res. 2016;102(2):213–216. doi: 10.1016/j.otsr.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Palmerini E., Ruggieri P., Angelini A., Boriani S., Campanacci D., Milano G.M., Cesari M., Paioli A., Longhi A., Abate M.E., Scoccianti G., Terzi S., Trovarelli G., Franchi A., Picci P., Ferrari S., Leopardi M.P., Pierini M. Denosumab in patients with aneurysmal bone cysts: a case series with preliminary results. Tumori. 2018;104(5):344–351. doi: 10.1177/0300891618784808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capanna R., Sudanese A., Baldini N., Campanacci M. Phenol as an adjuvant in the control of local recurrence of benign neoplasms of bone treated by curettage. Ital. J. Orthop. Traumatol. 1985;11(3):381–388. [PubMed] [Google Scholar]
- 17.Kececi B., Kucuk L., Isayev A., Sabah D. Effect of adjuvant therapies on recurrence in aneurysmal bone cysts. Acta Orthop. Traumatol. Turc. 2014;48(5):500–506. doi: 10.3944/AOTT.2014.14.0020. [DOI] [PubMed] [Google Scholar]
- 18.Pietschmann M.F., Dietz R.A., Utzschneider S., Baur-Melnyk A., Jansson V., Durr H.R. The influence of adjuvants on local recurrence rate in giant cell tumour of the bone. Acta Chir. Belg. 2010;110(6):584–589. [PubMed] [Google Scholar]
- 19.Nithyananth M., Priscilla A.J., Boopalan P.V., Titus V.T., Lee V.N. Time required for effective action of phenol against giant cell tumour cells. J. Orthop. Surg. (Hong Kong) 2014;22(1):104–107. doi: 10.1177/230949901402200126. [DOI] [PubMed] [Google Scholar]
- 20.Lin W.C., Wu H.T., Wei C.J., Chang C.Y. Aneurysmal bone cyst arising from fibrous dysplasia of the frontal bone (2004:2b) Eur. Radiol. 2004;14(5):930–932. doi: 10.1007/s00330-003-2181-4. [DOI] [PubMed] [Google Scholar]
- 21.Wang E.H., Marfori M.L., Serrano M.V., Rubio D.A. Is curettage and high-speed burring sufficient treatment for aneurysmal bone cysts? Clin. Orthop. Relat. Res. 2014;472(11):3483–3488. doi: 10.1007/s11999-014-3809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topouchian V., Mazda K., Hamze B., Laredo J.D., Pennecot G.F. Aneurysmal bone cysts in children: complications of fibrosing agent injection. Radiology. 2004;232(2):522–526. doi: 10.1148/radiol.2322031157. [DOI] [PubMed] [Google Scholar]
- 23.Varshney M.K., Rastogi S., Khan S.A., Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin. Orthop. Relat. Res. 2010;468(6):1649–1659. doi: 10.1007/s11999-009-1144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiels W.E., 2nd, Beebe A.C., Mayerson J.L. Percutaneous doxycycline treatment of juxtaphyseal aneurysmal bone cysts. J. Pediatr. Orthop. 2016;36(2):205–212. doi: 10.1097/BPO.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 25.Roux S., Massicotte M.H., Huot Daneault A., Brazeau-Lamontagne L., Dufresne J. Acute hypercalcemia and excessive bone resorption following anti-RANKL withdrawal: case report and brief literature review. Bone. 2019;120:482–486. doi: 10.1016/j.bone.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Basarir K., Piskin A., Guclu B., Yildiz Y., Saglik Y. Aneurysmal bone cyst recurrence in children: a review of 56 patients. J. Pediatr. Orthop. 2007;27(8):938–943. doi: 10.1097/bpo.0b013e31815a5fd3. [DOI] [PubMed] [Google Scholar]
- 27.Brosjo O., Pechon P., Hesla A., Tsagozis P., Bauer H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop. 2013;84(5):502–505. doi: 10.3109/17453674.2013.850013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dormans J.P., Hanna B.G., Johnston D.R., Khurana J.S. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin. Orthop. Relat. Res. 2004;421:205–211. doi: 10.1097/01.blo.0000126336.46604.e1. [DOI] [PubMed] [Google Scholar]
- 29.Erol B., Topkar M.O., Caliskan E., Erbolukbas R. Surgical treatment of active or aggressive aneurysmal bone cysts in children. J. Pediatr. Orthop. B. 2015;24(5):461–468. doi: 10.1097/BPB.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 30.Flont P., Kolacinska-Flont M., Niedzielski K. A comparison of cyst wall curettage and en bloc excision in the treatment of aneurysmal bone cysts. World J. Surg. Oncol. 2013;11:109. doi: 10.1186/1477-7819-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibbs C.P., Jr., Hefele M.C., Peabody T.D., Montag A.G., Aithal V., Simon M.A. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J. Bone Joint Surg. Am. 1999;81(12):1671–1678. doi: 10.2106/00004623-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Kurucu N., Akyuz C., Ergen F.B., Yalcin B., Kosemehmetoglu K., Ayvaz M., Varan A., Aydin B., Kutluk T. Denosumab treatment in aneurysmal bone cyst: Evaluation of nine cases. Pediatr. Blood Cancer. 2018;65(4) doi: 10.1002/pbc.26926. [DOI] [PubMed] [Google Scholar]
- 33.Mankin H.J., Hornicek F.J., Ortiz-Cruz E., Villafuerte J., Gebhardt M.C. Aneurysmal bone cyst: a review of 150 patients. J. Clin. Oncol. 2005;23(27):6756–6762. doi: 10.1200/JCO.2005.15.255. [DOI] [PubMed] [Google Scholar]
- 34.Peeters S.P., Van der Geest I.C., de Rooy J.W., Veth R.P., Schreuder H.W. Aneurysmal bone cyst: the role of cryosurgery as local adjuvant treatment. J. Surg. Oncol. 2009;100(8):719–724. doi: 10.1002/jso.21410. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez A.R., Stanton R.P. Aneurysmal bone cyst in 29 children. J. Pediatr. Orthop. 2002;22(4):533–539. [PubMed] [Google Scholar]
- 36.Rastogi S., Varshney M.K., Trikha V., Khan S.A., Choudhury B., Safaya R. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol. A review of 72 cases with long-term follow-up. J. Bone Joint Surg. Br. 2006;88(9):1212–1216. doi: 10.1302/0301-620X.88B9.17829. [DOI] [PubMed] [Google Scholar]
- 37.Schulte M., Sarkar M.R., Von Baer A., Schultheiss M., Suger G., Hartwig E. Therapy of aneurysmal bone cyst. Unfallchirurg. 2000;103(2):115–121. doi: 10.1007/s001130050022. [DOI] [PubMed] [Google Scholar]
- 38.Shooshtarizadeh T., Movahedinia S., Mostafavi H., Jamshidi K., Sami S.H. Aneurysmal bone cyst: an analysis of 38 cases and report of four unusual surface ones. Arch. Bone Jt. Surg. 2016;4(2):166–172. [PMC free article] [PubMed] [Google Scholar]
- 39.Solooki S., Keikha Y., Vosoughi A.R. Can ethanol be used as an adjuvant to extended curettage in order to reduce the recurrence rate of aneurysmal bone cyst? Rev. Bras. Ortop. 2017;52(3):349–353. doi: 10.1016/j.rboe.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.