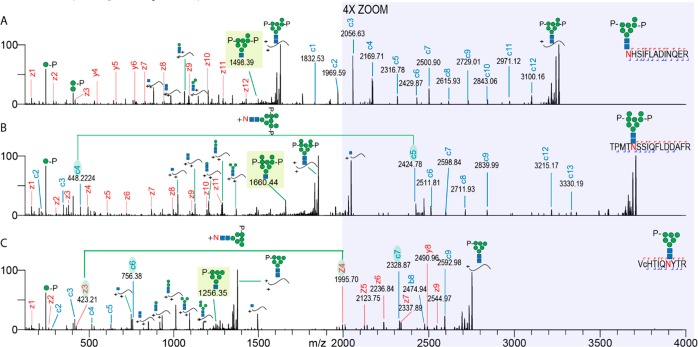
Figure 1.
Extended EThcD range results in the detection of additional fragment ions, increasing the sequence coverage of M6P glycopeptides. The EThcD fragmentation spectra of three different glycopeptides are shown. (A) GlcNAc2Man7P glycoform of palmitoyl-protein thioesterase 1, (B) GlcNAc2Man8PP glycoform of N-acetylglucosamine-6-sulfatase, and (C) GlcNAc2Man6P glycoform of cathepsin Z. Fragment ions are annotated and color coded (z/y red and c blue). Peptide sequences with corresponding glycoforms are depicted in the top right corner of each spectrum. The range from 2000 to 4000 m/z is shown in the shaded region magnified by a factor of 4. Green lines and shaded c/z ions connect fragment ion pairs containing the asparagine + intact glycan mass increment. Shaded glycan fragment ions represent signature M6P EThcD cleavage ions, facilitating confident glycan composition annotation. Lower case c in the peptide sequence indicates a carbamidomethylated cysteine.