Figure 1.
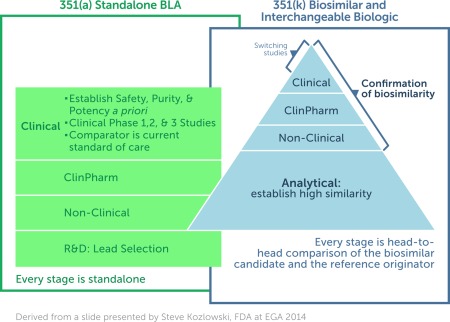
A representation of the data requirements for the development of a standalone biologic [351(a)] as compared to those expected for a biosimilar/interchangeable biologic [351(k)]. The concept of biosimilarity is fundamentally different from that applied to an originator biologic where safety, purity, and potency must be established a priori.
