Figure 2.
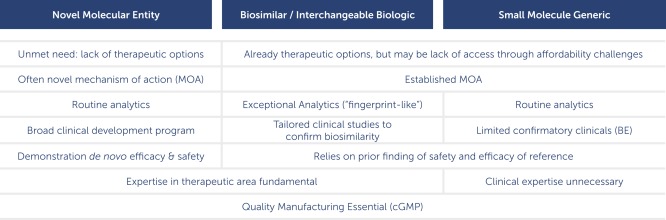
A comparison of the regulatory expectations for a novel molecular entity (drug or biologic), for a classic small molecule generic, and for a biosimilar/interchangeable biologic. The latter shares attributes of the other two.

A comparison of the regulatory expectations for a novel molecular entity (drug or biologic), for a classic small molecule generic, and for a biosimilar/interchangeable biologic. The latter shares attributes of the other two.