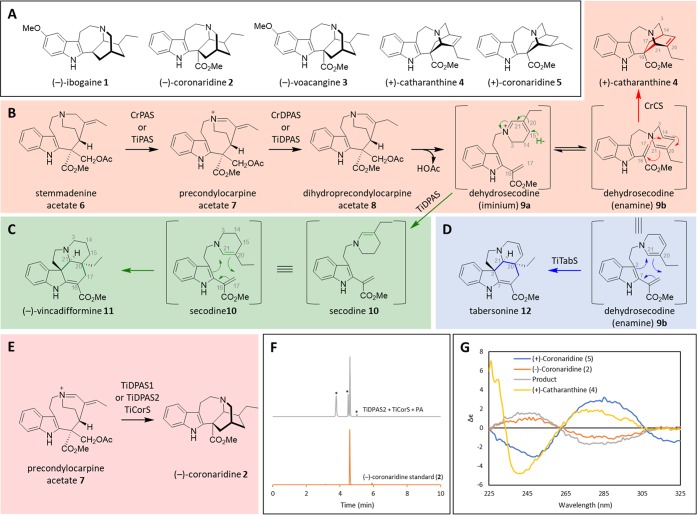
Figure 1.
(+) and (−)-Iboga alkaloids. (A) Anti-addiction agents (−)-ibogaine (1) and (−)-voacangine (3) are antipodal to (+)-catharanthine (4), a precursor to the anticancer drug vincristine. (B) Biosynthesis of (+)-catharanthine (4). It is assumed that dehydrosecodine iminium 9a is initially formed, and it then tautomerizes to the enamine form 9b. (C) Biosynthesis of (−)-vincadifformine (11). (D) Biosynthesis of (−)-tabersonine (12). (E) Biosynthesis of the reduced iboga alkaloid (−)-coronaridine (2) directly from precondylocarpine acetate (7). (F) LC-MS chromatogram showing formation of (−)-coronaridine (2) after incubation of precondylocarpine acetate (7) (50 μM) with TiDPAS2 (1 μM), TiCorS (5 μM), and NADPH (8 equiv). Peaks marked with an asterisk are uncharacterized side products (m/z 339) that decomposed during isolation attempts. (G) CD spectra of enzymatically produced coronaridine compared to authentic standards.