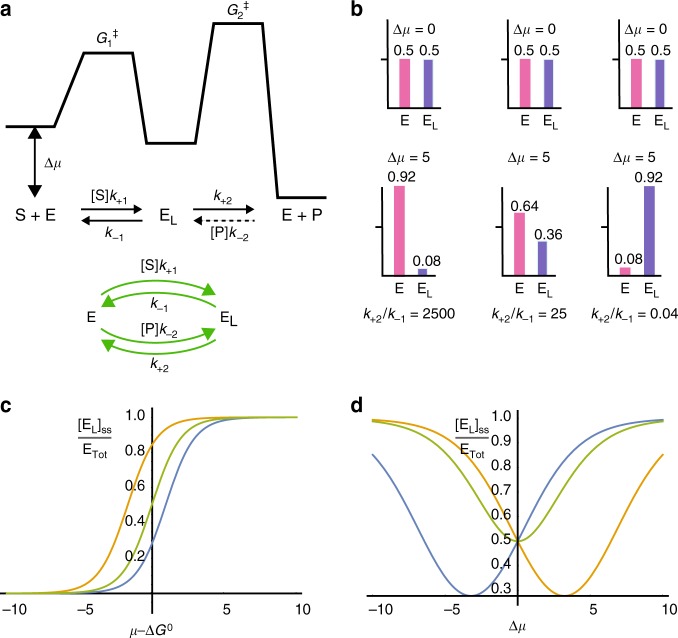
Fig. 2.
Kinetic asymmetry and Michaelis–Menten enzymes. a Energy profile for a Michaelis–Menten mechanism for catalysis, and two equivalent ways of writing the mechanism. An important quantity for determining the non-equilibrium behavior of the enzyme is the difference in transition state free-energies, , which can be expressed in terms of the rate constants as . This ratio does not depend on either μ or Δμ. b Bar graphs illustrating the equilibrium () distribution, where the kinetic asymmetry has no role, and the non-equilibrium () steady-state distributions that strongly depend on the kinetic asymmetry . c, d The concentrations of substrate (S) and product (P) are taken as constant (i.e., chemo-stated) in all calculations, and can be written in terms of the chemical potential using activity coefficients and . In ideal solutions both activity coefficients are ~ 1 in the units of concentration in which [S] and [P] are specified. Two types of enzyme adaptation: c “equilibrium” adaptation where the binding is based on the reference chemical potential , with , , and (orange); (green); and (blue). d Non-equilibrium adaptation governed by Δμ plotted at fixed . The binding is controlled by and by the kinetic asymmetry ()