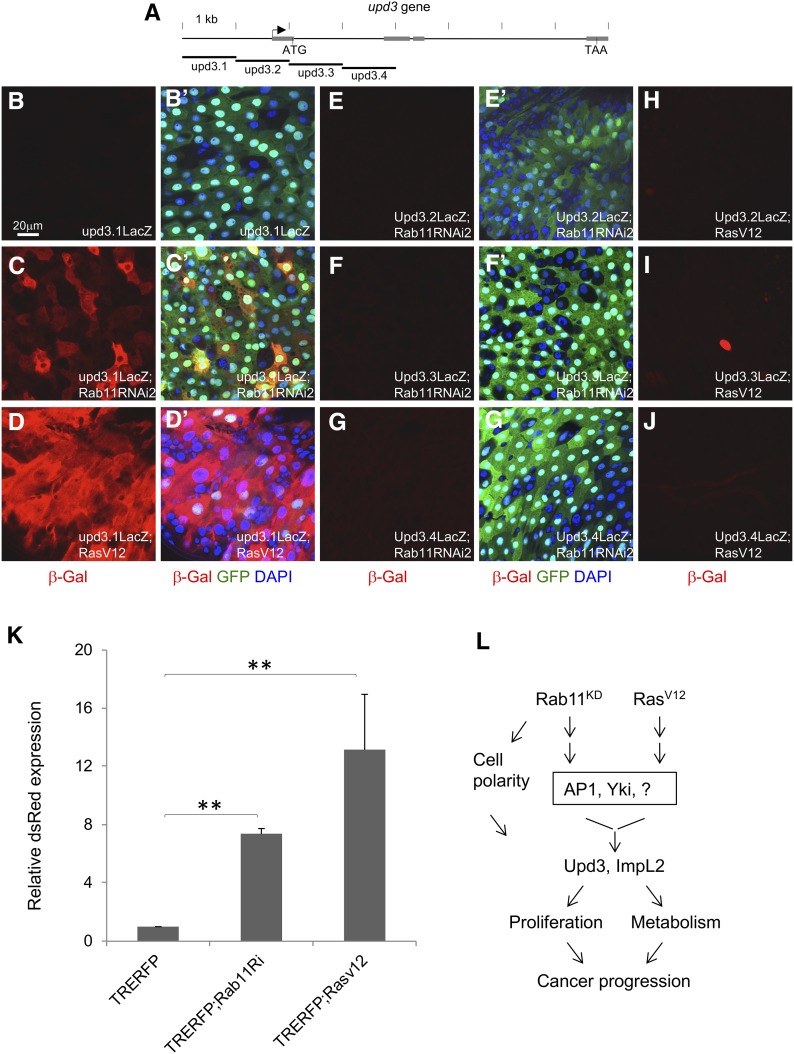
Figure 5.
Multiple regulatory components act downstream of Rab11 and RasV12. (A) A diagram showing the genomic locus of upd3. The arrow indicates predicted transcription start site. The gray boxes are exons, and the start and stop codons as ATG and TAA, respectively, are indicated. The four genomic elements labeled upd3.1-upd3.4, each containing ∼1 kb of DNA, were used to generate transgenic lacZ reporter flies (Jiang et al. 2009). (B–J) Each of the four reporters was crossed with either Rab11 RNAi or RasV12 overexpression flies, and then with the Myo1Ats > GFP driver. The resulting progenies were used for temperature shift for 3 days and then gut dissection. Antibodies for β-galactosidase were used for tissue staining and shown as read in the images. (B’–G’) are same corresponding images showing the 3-color combination. (H–J) No corresponding 3-color combinations are shown here because there was no significant β-galactosidase staining and they were similar to those shown in E’–G’. The color blue is DAPI, green is GFP and red is β-galactosidase. (K) Flies containing the AP1 reporter TRE-RFP were crossed to Rab11 RNAi or RasV12 flies and the resulting progenies were crossed with the Myo1Ats > GFP driver. After temperature shift for 3 days, the flies were used for gut dissection, RNA extraction, and qPCR quantification of dsRed mRNA expression. The expression in the control flies was set as 1, and the other samples were plotted as fold increase. (L) A diagram summarizes the results presented in this report. The working model is that loss of Rab11 function or activation of Ras can lead to activation of AP1, Yki, and other possible transcriptional regulators. The pathways converge onto the promoter elements of target genes including upd3 and ImpL2 to control their expression in the midgut. Upd3 expression increases intestine stem cell proliferation and ImpL2 expression reduced the metabolism of surrounding tissue, and together with the loss of cell polarity may promote cancer progression.