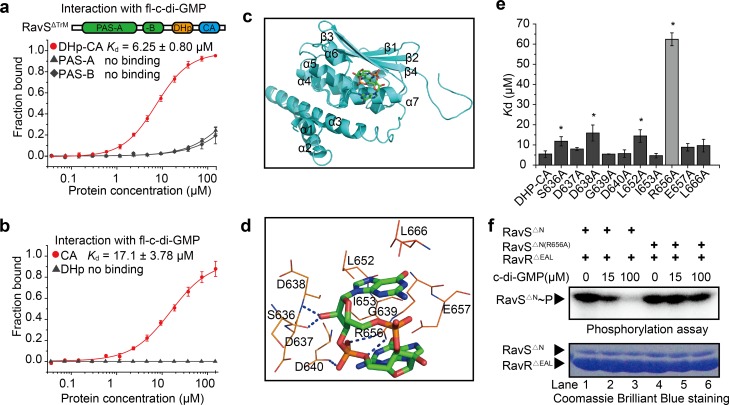
Fig 6. Arg656 in the CA domain of RavS is a key residue for c-di-GMP binding.
(a, b) c-di-GMP binds to the DHp-CA domain of RavS. PAS-A, PAS-B and DHp-CA in (a), and DHp and CA domains of RavS in (b), were purified and the interaction with c-di-GMP was measured by MST. Each assay was repeated three times. Standard deviations are shown. (c) Molecular docking model of the interaction between c-di-GMP and DHp-CA of RavS. c-di-GMP is shown as a ball-and-stick model and DHp-CA is shown in ribbon representation. (d) Schematic view of the predicted docking site between c-di-GMP and DHp-CA. Potential hydrogen bonds are indicated as blue dashed lines. Autodock software was used to predict the interaction. (e) Effect of amino acid substitutions on RavS–c-di-GMP binding. Ten recombinant proteins of the DHp-CA region, each containing a substitution of a putative essential residue involved in c-di-GMP binding, were used in the MST assay to quantify the Kd value of RavS–c-di-GMP binding. fl-c-di-GMP was used in the MST assay. Each experiment was repeated three times. (f) Substitution of Arg656 of RavS resulted in the loss of RavS-RavR phosphotransfer upon stimulation by c-di-GMP. A total of 5 μM RavSΔN or RavSΔN(R656A) was phosphorylated by 10 μCi [γ-32P]ATP for 15 min in the absence or presence of 15 or 100 μM c-di-GMP, and then 15 μM RavRΔEAL was added and incubated for 30 min at 25°C. The reactions were terminated with 5× SDS loading buffer and the products were separated by 12% SDS-PAGE, exposed to a phosphor screen and analysed by Typhoon FLA7000. The experiment was repeated three times.