Abstract
Skin reaction may develop at the site of vaccine administration. A 54-year-old woman who developed a cellular blue nevus at the site of the combined tetanus, diphtheria, and acellular pertussis (Tdap) vaccine injection four years prior to presentation is described. In addition to blue nevus, other reactions at combined tetanus, diphtheria, and pertussis vaccine injection sites include abscess, deep reactive nodular infiltrates of mixed inflammation, and necrotizing granuloma. In conclusion, blue nevus can be added to the list of cutaneous events that can occur at Tdap vaccination sites.
Keywords: blue, cutaneous, deltoid, diphtheria, nevus, papule, pertussis, tetanus, vaccination, vaccine
Introduction
Adverse cutaneous events can occur in vaccine injection sites [1]. These include inflammatory reactions and neoplasms. We describe a woman who developed a cellular blue nevus at the site of previous tetanus, diphtheria, and acellular pertussis (Tdap) injection. Cutaneous reactions to combined tetanus, diphtheria, and acellular pertussis vaccines and adverse skin reactions and neoplasms appearing in previous sites of vaccinations are also reviewed.
Case presentation
A 54-year-old woman presented with a new lesion on her left arm. Four years prior to presentation, an acquaintance of the patient developed pertussis, which prompted the patient to seek immunization. The patient had not been vaccinated against pertussis in childhood. Based on the Center for Disease Control age recommendations, Tdap was administered on her left deltoid; the patient subsequently developed a new lesion over the previous site of the vaccine administration.
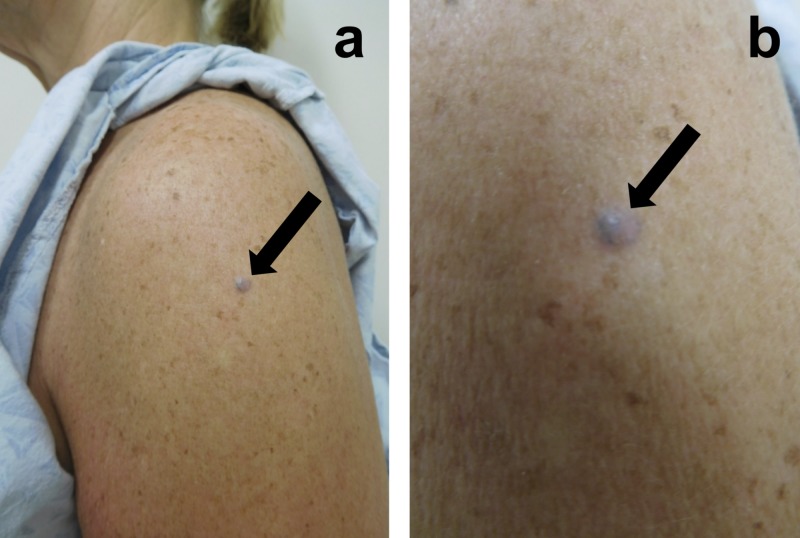
Cutaneous examination revealed a 5 x 5 mm papule on her prior Tdap vaccination site (Figure 1). A punch biopsy was performed. Microscopic examination showed ovoid melanocytes in the dermis. Collagen was trapped between the melanocytes and the surrounding fibrous stroma.
Figure 1. Cellular blue nevus appearing at the site of previous tetanus, diphtheria, and acellular pertussis (Tdap) vaccination.
Distant (a) and closer (b) views of the deltoid area of the left arm of a 54-year-old woman showing a cellular blue nevus (black arrow) that developed at the site of a previous tetanus, diphtheria, and acellular pertussis (Tdap) vaccination that she received four years earlier.
Correlation of the clinical history, lesion morphology, and pathology established the diagnosis of a cellular blue nevus developing at the site of the Tdap vaccination. The residual lesion was excised. There has been no recurrence.
Discussion
The first vaccination against pertussis, also commonly called “whooping cough,” was formulated using killed whole-cell Bordetella pertussis bacilli and licensed in 1914 [2]. Currently, there is no isolated vaccination for pertussis. Combination vaccines containing diphtheria toxoids, tetanus toxoids, and chemically-inactivated whole-cell pertussis were introduced as DTP in the 1940s; the acronym DTP was based on the first letter of each respective component. Subsequently, based on concerns regarding the adverse reactions to inactivated whole-cell pertussis, researchers developed an acellular vaccination for pertussis immunity; this vaccination came into wider use in the 1990s in the United States as the combined diphtheria, tetanus, and acellular pertussis vaccination (DTaP) [2-3].
The DTaP vaccination did not maintain adequate long-term humoral immunity against pertussis [4]. Therefore, a booster formulation of tetanus toxoid and smaller concentrations of the diphtheria toxoid and acellular pertussis received the approval from the Federal Drug Administration in 2005 as Tdap. Both DTaP and Tdap are currently in use. According to the United States Center for Disease Control, in children under seven years of age, DTaP is recommended, whereas for older children and for adults who have never been vaccinated, the Tdap is recommended [5].
Numerous cutaneous reactions have been observed at the sites of both live and attenuated vaccinations. These reactions include not only inflammatory reactions such as lichenoid and granulomatous dermatosis, but also neoplasms such as basal cell carcinoma, dermatofibrosarcoma protuberans, and squamous cell carcinoma (Table 1, Table 2) [1]. Skin-related reactions specific to diphtheria, tetanus, and pertussis vaccination include deep reactive nodular infiltrates of mixed inflammation, Mycobacterium tuberculosis abscess, and necrotizing granuloma (Table 3) [6-7]. The development of benign or malignant neoplasms at prior sites of vaccination may be coincidental.
Table 1. Benign cutaneous lesions associated with vaccination sites.
BCG = Bacillus Calmette-Guerin vaccine; DRNIMI = Deep reactive nodular infiltrates of mixed inflammation; DTaP-IPV-Hib = Diphtheria, tetanus, pertussis, polio, Haemophilus influenzae Type B vaccine; DPT = Diphtheria, whole-cell pertussis, and tetanus vaccine; DTP = Diphtheria, tetanus, and whole-cell pertussis vaccine; ESM = Early summer meningitis vaccine; Flu = Influenza vaccine; HepB = Hepatitis B vaccine; PLAPR = Papulonodular lichenoid and pseudolymphomatous reaction; Pne = Pneumococcal vaccine; Sp = Smallpox vaccine; Td = Tetanus and diphtheria vaccine; Tdap-IPV = Tetanus, diphtheria, pertussis, and polio vaccine; Tet = Tetanus vaccine; VZV = Varicella-zoster virus vaccine
aRobust take is a skin reaction at the site of administration greater than 7.5 cm in size with symptoms of joint pain, swelling, and warmth.
| Cutaneous lesions | Vaccination |
| Abscess or cellulitis | BCG, Pne |
| Allergic contact dermatitis | Sp |
| Angiolymphoid hyperplasia with eosinophils | Tet |
| Blistering | BCG |
| Churg-Strauss vasculitis | HepB |
| DRNIMI | DTP |
| Dermatitis, chronic | Sp |
| Dermatofibroma | Sp |
| Epithelial cyst | BCG |
| Erythema | BCG, HepB, Pne, VZV |
| Fixed drug eruption | BCG |
| Foreign body granuloma (non-necrotizing) | BCG |
| Granuloma annulare | BCG, HepB, Td, Tet |
| Granuloma (delayed) | BCG |
| Herpes simplex virus infection | Sp |
| Indurated erythematous plaque (pseudoplymphoma) | Tet |
| Inflammatory reaction, localized | Sp |
| Itching granuloma | Pne, DTaP-IPV-Hib |
| Isotopic response to patch testing | BCG |
| Keloid | BCG, HepB, Sp |
| Lichenoid dermatitis | Pne |
| Lupus erythematosus (discoid) | Sp |
| Lupus vulgaris (cutaneous tuberculosis) | BCG |
| Lymphadenopathy (suppurative) | BCG |
| Mastocytoma | HepB |
| Mycobacterium chelonae abscess | Tdap-IPV |
| Mycobacterium tuberculosis abscess | DPT |
| Myxedematous infiltration, diffuse | Sp |
| Necrobiotic granuloma | HepB |
| Necrotizing granulomatous reaction | BCG, DTP |
| Nevus sebaceous | Sp |
| Nodules | HepB |
| Papular tuberculids | BCG |
| PLAPR | HepB |
| Pigmentation | Sp |
| Pilomatricoma | BCG |
| Post scab lesions | Sp |
| Progressive vaccinia | Sp |
| Psoriasis | BCG, Flu |
| Pyogenic infections | Sp |
| Robust takea | Sp |
| Sarcoidosis (juvenile) | BCG |
| Subcutaneous nodule (sterile abscess) | DTaP-IPV-Hib |
| Subcutaneous nodule (pseudolymphoma) | ESM, HepB, VZV |
| Sweet’s syndrome | BCG, Flu, Pne, Sp |
| Tufted angioma | BCG |
| Ulceration | BCG |
| Ulceration during Kawasaki disease | BCG |
| Vasculitis (ulcerating) | BCG |
| Zosteriform eruption | VZV |
Table 2. Neoplasms associated with vaccination sites.
BCG = Bacillus Calmette-Guerin vaccine; Lsh = Leishmaniasis vaccine; Pne = Pneumococcal vaccine; Sp = Smallpox vaccine; TPY = Tetanus, plague, yellow fever vaccine
| Cutaneous lesions | Vaccination |
| Basal cell carcinoma | BCG, Sp |
| Dermatofibrosarcoma protuberans | Lsh, Sp, TPY, Travel immunization |
| Fibrosarcoma | Sp |
| Keratoacanthoma | Pne, Sp |
| Malignant fibrous histiocytoma | Sp |
| Melanoma | Sp |
| Squamous cell carcinoma | BCG, Sp |
Table 3. Combined tetanus, diphtheria, and pertussis vaccine site reactions.
CR = Current report; DRNIMI = Deep reactive nodular infiltrates of mixed inflammation; DPT = Diphtheria, whole-cell pertussis, and tetanus vaccine; DTP = Diphtheria, tetanus, and whole-cell pertussis vaccine; Tdap = Tetanus, diphtheria, and acellular pertussis vaccine
aThe cited reference refers to the combined diphtheria, whole-cell pertussis, and tetanus vaccination as “DPT,” which is an alternative acronym for DTP.
Our patient developed a cellular blue nevus on the site of the injection after she received a Tdap vaccination [8]. To the best of our knowledge, she is the only person who has developed a vaccination site-related blue nevus. In addition, we are not aware of any other individuals developing a melanocytic lesion at the site of Tdap vaccination.
A postulated pathogenesis for the formation of a new cutaneous disease at a site of prior cutaneous insult has been attributed to the creation of an “immunocompromised district”-a localized site of immune destabilization in the skin leading to increased risk of developing dermatoses [9]. Through this potential mechanism, vaccination administration may allow the site to be more prone to developing a wide array of skin conditions. The appearance of our patient’s cellular blue nevus following localized skin trauma from the vaccination may be the etiology of this phenomenon.
The administration of vaccinations is extremely common. The development of cutaneous adverse reactions at the site of vaccination is a rare occurrence. Although the rate for cutaneous adverse reactions at the vaccination sites remains to be established, the estimated rate would be very low based on the published literature of these events.
Conclusions
Adverse events may occur in local vaccination sites. Our patient developed a cellular blue nevus at the site of the Tdap vaccine administration. The development of a melanocytic nevus at the site of Tdap vaccination has not been previously described as a post-vaccination sequela. We suggest that this vaccination site-related tumor may have resulted from the creation of a local immunocompromised district in the skin following Tdap vaccination.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Injection site lichenoid dermatitis following pneumococcal vaccination: report and review of cutaneous conditions occurring at vaccination sites. Cohen PR. Dermatol Ther. 2016;6:287–298. doi: 10.1007/s13555-016-0105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Licensed pertussis vaccines in the United States. History and current state. Klein NP. Hum Vaccin Immunother. 2014;10:2684–2690. doi: 10.4161/hv.29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pertussis: history of the disease and current prevention failure. Kuchar E, Karlikowska-Skwarnik M, Han S, Nitsch-Osuch A. Adv Exp Med Biol. 2016;934:77–82. doi: 10.1007/5584_2016_21. [DOI] [PubMed] [Google Scholar]
- 4.Declining pertussis incidence in Sweden following the introduction of acellular pertussis vaccine. Olin P, Gustafsson L, Barreto L, et al. Vaccine. 2003;21:2015–2021. doi: 10.1016/s0264-410x(02)00777-6. [DOI] [PubMed] [Google Scholar]
- 5.Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Liang JL, Tiwari T, Moro P, et al. MMWR Recomm Rep. 2018;62:1–44. doi: 10.15585/mmwr.rr6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Injection site abscess due to Mycobacterium tuberculosis following DPT vaccination. Dixit R, Dixit K, Patil CB, Pawar KS. https://www.ncbi.nlm.nih.gov/pubmed/25241575. Indian J Tuberc. 2014;61:246–249. [PubMed] [Google Scholar]
- 7.Postimmunization (vaccination) injection-site reactions. A report of four cases and review of the literature. Miliauskas JR, Mukherjee T, Dixon B. https://www.ncbi.nlm.nih.gov/pubmed/8470766. Am J Surg Pathol. 1993;17:516–524. [PubMed] [Google Scholar]
- 8.Blue nevi and variants: an update. Zembowicz A, Phadke PA. https://www.ncbi.nlm.nih.gov/pubmed/21366456. Arch Pathol Lab Med. 2011;135:327–336. doi: 10.5858/2009-0733-RA.1. [DOI] [PubMed] [Google Scholar]
- 9.The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. Ruocco V, Brunetti G, Puca RV, Ruocco E. J Eur Acad Dermatol Venereol. 2009;23:1364–1373. doi: 10.1111/j.1468-3083.2009.03345.x. [DOI] [PubMed] [Google Scholar]