Abstract
Background and aims
Diabetes is an independent risk factor for carotid artery stenosis (CAS). Fatty acid synthase (FAS), an essential de novo lipogenesis enzyme, has increased activity in the setting of diabetes that leads to altered lipid metabolism. Circulating FAS (cFAS) was recently observed in the blood of patients with hyperinsulinemia and cancer. We thought to evaluate the origin of cFAS and its role in diabetes-associated CAS.
Methods
Patients with diabetes and no diabetes, undergoing carotid endarterectomy (CEA) for CAS, were prospectively enrolled for collection of plaque and fasting serum. FPLC was used to purify lipoprotein fractions, and ELISA was used to quantify cFAS content and activity. Immunoprecipitation (IP) was used to evaluate the affinity of cFAS to LDL-ApoB.
Results
Patients with CAS had higher cFAS activity (p<0.01), and patients with diabetes had higher cFAS activity than patients with no diabetes (p<0.05). cFAS activity correlated with serum glucose (p=0.03, r2=0.35), and cFAS content trended with plaque FAS content (p=0.06, r2=0.69). cFAS was predominantly in LDL cholesterol fractions of patients with CAS (p<0.001), and IP of cFAS demonstrated pulldown of ApoB. Similar to patients with diabetes, db/db mice had highest levels of serum cFAS (p<0.01), and fasL−/− (tissue-specific liver knockdown of FAS) mice had the lowest levels of cFAS (p<0.001).
Conclusions
Serum cFAS is higher in patients with diabetes and CAS, appears to originate from the liver, and is LDL cholesterol associated. We postulate that LDL may be serving as a carrier for cFAS that contributes to atheroprogression in carotid arteries of patients with diabetes.
Keywords: Fatty acid synthase, Carotid artery stenosis, Serum biomarker, Diabetes, Lipoprotein
1. INTRODUCTION
Diabetes is a strong independent risk factor for cerebrovascular disease.[1, 2] At least 30% of ischemic strokes occur in a carotid artery distribution, and are a consequence of atherosclerotic occlusive disease leading to critical carotid artery stenosis (CAS).[3, 4] Diabetes and serum cholesterol are known predictors for carotid atheroprogression,[3] and patients with diabetes are at particularly higher risk of developing ischemic stroke manifestations of the disease.[5, 6] Although little mechanistic information exists on the effect of diabetes on atheroprogression in the carotid artery,[7] it is assumed that chronic hyperglycemia, altered metabolism, and abnormal serum lipid concentrations can affect the incidence and severity of atherosclerosis in patients with diabetes.[8]
Fatty Acid Synthase (FAS) is an essential multi-enzyme, 273kDa polypeptide dimer complex that catalyzes the endogenous de novo synthesis of saturated fatty acids (SFAs) from simple molecular precursors such as acyl-CoA and malonyl-CoA.[9, 10] In the setting of diabetes there is increased FAS gene expression and enzyme activity,[11] and a 10-fold increase in hepatic FAS-mediated lipogenesis.[9, 12] Conversely, treatment of high-fructose diet-fed db/db mice with FAS inhibitors decreases hepatic lipid accumulation and hepatic fatty acid oxidation.[13] Since FAS is regulated by insulin, glucagon, glucose, and dietary fats, this presumed soluble cytoplasmic protein is considered to play important roles in the pathogenesis of disorders associated with chronic diabetes.
Recently, FAS was also identified in the blood of patients with unique pathologies.[14] Circulating FAS (cFAS) is detected and elevated in the blood stream of patients with various types of advanced cancer,[15, 16] as well as in patients with age adjusted obesity-induced insulin resistance.[17] Given these findings and the already established role of tissue FAS in producing the metabolic building blocks for complex saturated lipids, triglycerides and lipoproteins (such as VLDL and LDL),[18] we thought to evaluate the levels of cFAS in patients with diabetes and high-grade CAS. We also thought to evaluate the potential tissue origin of cFAS to help explain the presence of this large homo-dimer protein in the blood stream.
2. MATERIALS AND METHODS
2.1. Human study patient selection and serum preparation
From December 2016 to December 2017, an equal number of patients with diabetes and no diabetes, undergoing carotid endarterectomy (CEA) for high-grade (>70%) asymptomatic or symptomatic CAS, were prospectively enrolled for the collection of carotid artery plaque tissue as well as preoperative fasting serum. Additionally, another cohort of younger ‘healthy’ patients, with no history of arterial occlusive disease, diabetes, or a history of current smoking was simultaneously recruited to obtain control fasting serum. This study was performed using an institutional review board-approved study protocol that conforms to the ethical guidelines of the 1975 Declaration of Helsinki. All study patients provided written informed consent prior to participation in the study.
Fresh human serum aliquots were collected from fasting (at least 12 hours) study participants and concentrated using a 100kDa ultrafiltration centrifuge tube (ThermoFisher Scientific, Waltham, MA), at 15,000g, for 15 minutes. A resultant minimum volume of 250 μL of concentrated serum was collected for each patient. The protein concentration of the concentrated human serum was assessed using a Bradford Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA).
2.2. Fast protein liquid chromatography (FPLC)
Human serum was fractionated using FPLC (Akta FPLC; GE Healthcare) using two tandem Superose 6 Increase 10/300 GL columns. Phosphate buffered saline (PBS; pH 7.4) eluent buffer was first passed through a 0.22 μm filter, at a rate of 0.2ml/min, to ensure equilibration of the columns. Serum sample aliquots of 500 μL were then injected into the columns. Elutions were then carried out at a flow rate of 0.2 mL/min. 72 fractions, 0.5 mL each, were collected in separate elutant glass tubes for subsequent cholesterol content analysis (Infinity Cholesterol Analysis; Fisher Scientific, Waltham, MA). 10 μL aliquots from each elutant fraction were then used to determine the discrete elution peaks for VLDL, LDL, and HDL lipoproteins.
2.4. Animals
Adult 8-week-old wildtype (+/+) C57BL6-J mice and db/db were commercially obtained (Jackson Laboratory, Bar Harbor, ME). Similarly aged, hepatocyte specific and skeletal muscle specific FAS knockdown mice (fasL−/− and fasM−/−, respectively) were generated as previously described.[19, 20] Briefly, mice were engineered with LoxP flanked fasn gene, and crossed with mice expressing Cre Recombinase either via an albumin promoter or alpha-actin muscle promoter. All murine housing, breeding, and experimental procedures were performed in accordance with national and local guidelines, and were approved by Institutional Animal Care and Use Committee.
2.5. cFAS enzyme linked immunosorbent assay (ELISA)
cFAS content in duplicate or triplicate serum and plaque samples was performed using a commercially available cFAS ELISA assay (Aviva Biosystems, San Diego, CA). cFAS enzymatic activity in serum and plaque was evaluated using a malonyl CoA-dependent NADPH depletion assay as previously described.[21]
2.6. Co-immunoprecipitation of cFAS and Apolipoprotein B (ApoB)
FPLC-derived peak LDL cholesterol fractions were collected for representative patients with diabetes. Co-immunoprecipitation of cFAS and ApoB was performed using a Protein A Sepharose bead pulldown assay. Refer to supplemental online material for method details.
2.7. Human and murine tissue lysis and Western blotting
Fresh human plaque tissue was grossly dissected and segmented into maximally (Max) and minimally (Min) diseased portions as previously described and validated.[22] Segments were then immersed in cold hypotonic non-detergent based lysis buffer (1M NaHCO3, 1M NaN3, 0.1 M PMSF, and Protease Inhibitor Cocktail Set III (Calbiochem, San Diego, CA)) for 30 minutes. Specimens were homogenized with high-speed rotational tissue homogenizer (Glas-Col, Terre Hute, IN), and centrifuged at 12,000 RPM for 5 minutes), and supernatants were collected for analysis.
Murine organ tissue were finely minced with a scissor and scalpel and immersed in 1× lysis buffer (Cell Signaling Technology, Danvers, MA)) and Protease Inhibitor Cocktail. Lysates were centrifuged, and supernatants collected for analysis.
Human carotid plaque, and murine liver and muscle tissue homogenates were evaluated with Western blot. Samples were standardized by protein concentration using a Bradford protein analysis assay (Bio-Rad, Hercules, CA). 30ug of protein from each sample was resolved on a 7% SDS-TrisAcetate-polyacrylamide gel and electrotransferred onto PVDF membranes. Membranes were then probed with an FAS antibody and GAPDH antibody (Cell Signaling Technologies, Danavers, MA), and Western blots were resolved with ECL kit and LI-COR imaging system.
2.8. Mass spectrometry
Modified Bligh-Dyer method [23] was performed to extract SFAs and unsaturated fatty acids (USFAs) from 50 μL of lysates of Max diseased carotid plaque segments. Refer to online supplemental methods for additional details.
2.9. Statistical analysis
All statistical analyses were carried out using GraphPad Prism (La Jolla, CA). Descriptive statistics are summarized as mean±SEM, unless otherwise indicated. Non-parametric Mann-Whitney tests were used for the following: to determine differences in demographic variables between patients with CAS and controls without CAS, differences in cFAS activity between CAS patients and controls, differences in cFAS content in CAS patient and controls, and FAS band densitometry between minimally versus maximally diseased carotid artery segments. Kruskal-Wallis tests were used to determine if significant differences existed in between patients with diabetes, no diabetes, and healthy patient controls in the following: patient demographic variables, cFAS serum content and activity, and peak isolate FPLC fractions. Student t-test was used to evaluate the differences in cFAS in the different the murine lines. Spearman correlation was used to evaluate relationships between cFAS, LDL, and serum glucose. All p≤0.05 were considered significant.
3. RESULTS
3.1. Patient clinical demographics
During the study period, we prospectively enrolled and evaluated 39 patients. This included 26 age and comorbidity matched patients (13 patients with type 2 diabetes and 13 patients without diabetes), who were undergoing CEA for confirmed high-grade CAS. An additional 13 patients with no history of cardiovascular disease, diabetes, or smoking were enrolled as controls. With the exception of history of hypertension and the use of Metformin in patients with diabetes, there were no other significant differences between the demographics of the patient with or without diabetes enrolled in this study (Table 1). In comparison, control patients were significantly younger, did not have any cardiovascular co-morbidities, and were mostly female (p<0.05).
Table 1:
Demographic variables of study patients
| Demographics | Diabetes and CEA (n=13) | No diabetes and CEA (n=13) | Healthy patients (n=13) |
|---|---|---|---|
| Diabetes | 13 | 0 | 0 |
| Gender (F) | 4 | 2 | 10 |
| Age | |||
| <50 | 0 | 0 | 12 |
| 50–60 | 2 | 2 | 1 |
| 61–70 | 5 | 4 | 0 |
| 71–80 | 4 | 7 | 0 |
| >80 | 2 | 0 | 0 |
| BMI | 4 | 3 | 4 |
| Current smoker | 0 | 0 | 1 |
| Symptomatic CAS | 6 | 5 | 0 |
| Asymptomatic CAS | 7 | 8 | 0 |
| History of prior stroke | 4 | 3 | 0 |
| Peripheral artery disease | 1 | 3 | 0 |
| Coronary artery disease | 2 | 5 | 0 |
| Hypertension* | 13 | 8 | 0 |
| Hyperlipidemia | 11 | 13 | 0 |
| Renal insufficiency | 0 | 0 | 0 |
| Heart arrhythmia | 3 | 1 | 0 |
| Valvular heart disease | 4 | 3 | 0 |
| Alcohol abuse | 0 | 0 | 0 |
| Medications | |||
| ACE inhibitor | 9 | 5 | 0 |
| Beta blocker | 7 | 7 | 0 |
| Aspirin | 9 | 12 | 0 |
| Other antiplatelet | 4 | 5 | 0 |
| Statin | 13 | 13 | 0 |
| Insulin | 0 | 0 | 0 |
| Metformin* | 9 | 0 | 0 |
p < 0.05, Mann-Whitney U test
3.2. cFAS content and activity is elevated in patients with diabetes and high-grade CAS
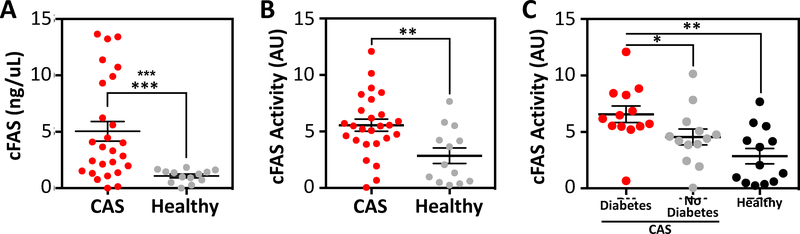
Compared to patients no CAS, patients with CAS had significantly elevated serum cFAS content (1.1 ± 0.2 versus 5.0 ± 0.9 respectively; p<0.001; Fig. 1A). Additionally, patients with CAS had higher cFAS enzyme activity compared to controls (5.6 ± 0.5 vs. 2.9 ± 0.7 pmoles of NADPH consumed, respectively; p<0.01; Fig. 1B). Patients with diabetes had significantly higher cFAS enzyme activity compared to patients with no diabetes (p<0.05) and control patients (p<0.01; Fig. 1C). No differences in serum cFAS activity were observed between patients with symptomatic versus asymptomatic CAS, or a history of coronary artery disease (CAD; Supplemental Fig. 1A and B). Similarly, no differences in serum cFAS activity were observed relative to gender, age, or BMI (Supplemental Fig. 1C–E).
Figure 1:
Serum cFAS is elevated in patients with CAS and diabetes.
(A) cFAS content (ng/μL) in the fasting serum of patients diagnosed with high-grade CAS and scheduled for CEA (n=26) was compared to fasting serum of patients who do not have CAS or other major cardiovascular co-morbidities (n=13). Patients with high-grade CAS demonstrated significantly higher cFAS content in their fasting serum compared to control patients. Control patients consistently demonstrated low serum cFAS content. ***p<0.001, Mann-Whitney. Error bars represent the SEM. (B) cFAS enzyme activity (arbitrary units, AU) was also evaluated between patients with high-grade CAS (n=26) and control patients (n=13). Patients with high-grade CAS demonstrated significantly higher cFAS enzyme activity compared to control patients **p<0.01, Mann-Whitney. Error bars represent the SEM. (C) Patients with diabetes and CAS (n=13) had the highest levels of cFAS enzyme activity compared to patients with no diabetes and CAS (n=13), and control patients (n=13). *p<0.05, **p<0.01, Kruskal-Wallis. Error bars represent the SEM.
3.3. cFAS correlates with serum glucose and LDL cholesterol
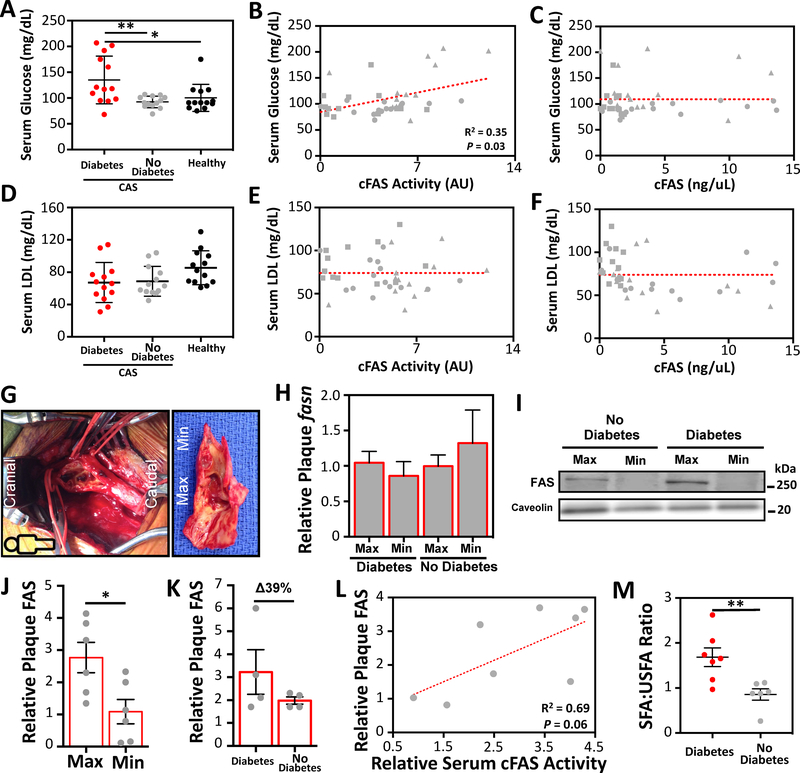
Serum cholesterol and glucose correlate with the severity of atherosclerotic disease in patients with diabetes, and LDL is a known independent risk factor for CAS[24] and stroke.[25] We therefore evaluated the relationship between serum cFAS, glucose, and LDL.
Patients with diabetes had significantly elevated fasting serum glucose compared to patients without diabetes (p<0.05; Fig. 2A). We observed that cFAS activity had a significant positive correlation with serum glucose (Spearman R2 = 0.35, p=0.03; Fig. 2B). cFAS content did not correlate with serum glucose (Fig. 2C). There was no significant difference in fasting serum LDL cholesterol between patients with and without diabetes and controls (Fig. 2D), and there was no meaningful correlation between cFAS content/activity and serum LDL cholesterol (Fig. 2E and F). Similarly, no differences in total cholesterol and serum triglycerides were observed between the study groups, and no correlations were observed between these variables and cFAS content/activity (Supplemental Fig. 2).
Figure 2:
Serum cFAS correlates with serum glucose and carotid plaque FAS and saturated fatty acid content.
(A) Serum cFAS activity and content were evaluated in patients with diabetes and CAS (n=13), patients with no diabetes and CAS (n=13), and controls with no diabetes and no CAS (n=13). Patients with diabetes and CAS have significantly higher serum glucose compared to patients with no diabetes and CAS. *p<0.05, Kruskal-Wallis. Error bars represent the SEM. cFAS activity (B) but not content (C) has a significant correlation with serum glucose (patient with diabetes and CAS, triangle; patient with no diabetes and CAS, square; patient control with no CAS, circle). r2, Spearman correlation. (D) No significant difference was observed in serum LDL between patients with and without diabetes, and control patients. (E and F) No correlation was observed between cFAS activity/content and LDL. (G) Open exposure of the right carotid artery bifurcation with the head towards the left (cranial) and the feet towards the right (caudal). Visible within the clamped carotid bifurcation is irregular ulcerated plaque. Extracted CEA plaque demonstrates a minimally (Min) diseased portion at the distal edge, and a maximally (Max) diseased portion at the carotid bifurcation region. (H) fasn RNA transcript between Max and Min diseased CEA portions was evaluated using RT-PCR. No differences were observed in fasn and between patients with and without diabetes. (I) FAS protein content in CEA plaque homogenates were evaluated by Western blot analysis. (J) Higher levels of FAS were observed in Max diseased plaque compared to Min diseased plaque (n=6 patients). *p<0.05, Mann-Whitney U test. Error bars represent the SEM. (K) FAS protein content in CEA plaque homogenates was evaluated using ELISA. Compared to patients with no diabetes, there was a 39% increase in FAS in the Max and Min CEA plaques of patients with diabetes. Error bars represent the SEM. (L) FAS protein content in CEA plaque demonstrated a moderate correlation with serum cFAS activity, but this difference was not statistically significant. r2, Spearman correlation; p=0.06. (M) The ratio of the relative content of total saturated fatty acid (SFA) to total unsaturated fatty acid (USFA) was significantly higher in the Max CEA plaques of patients with diabetes. **p<0.001, Kruskal-Wallis. Error bars represent the SEM.
3.4. FAS content is elevated in maximally diseased carotid plaque segments
Carotid plaque from patients enrolled in the study was collected and analyzed for FAS content in the maximally (Max) and minimally (Min) diseased plaque portions (Fig. 2G). No difference in fasn RNA transcript was observed in Max and Min diseased CEA plaque segments of patients with or without diabetes (Fig. 2H). However, we observed that Max diseased CEA plaque segments had 2.5 times higher FAS protein content (p<0.05; Fig. 2I and J). Patients with diabetes also demonstrated a 39% higher FAS content in Max diseased carotid segments compared to patients with no diabetes (p>0.05; Fig. 2K). The relative content of FAS in CEA plaque and serum cFAS activity in the same patients demonstrated a moderate but non-significant correlation (Spearman r2=0.69; p=0.06; Fig. 2L). There was also a higher content of SFAs in Max diseased CEA plaque segments of patients with diabetes (p<0.001; Fig. 2M).
3.5. cFAS is associated with LDL cholesterol and ApoB
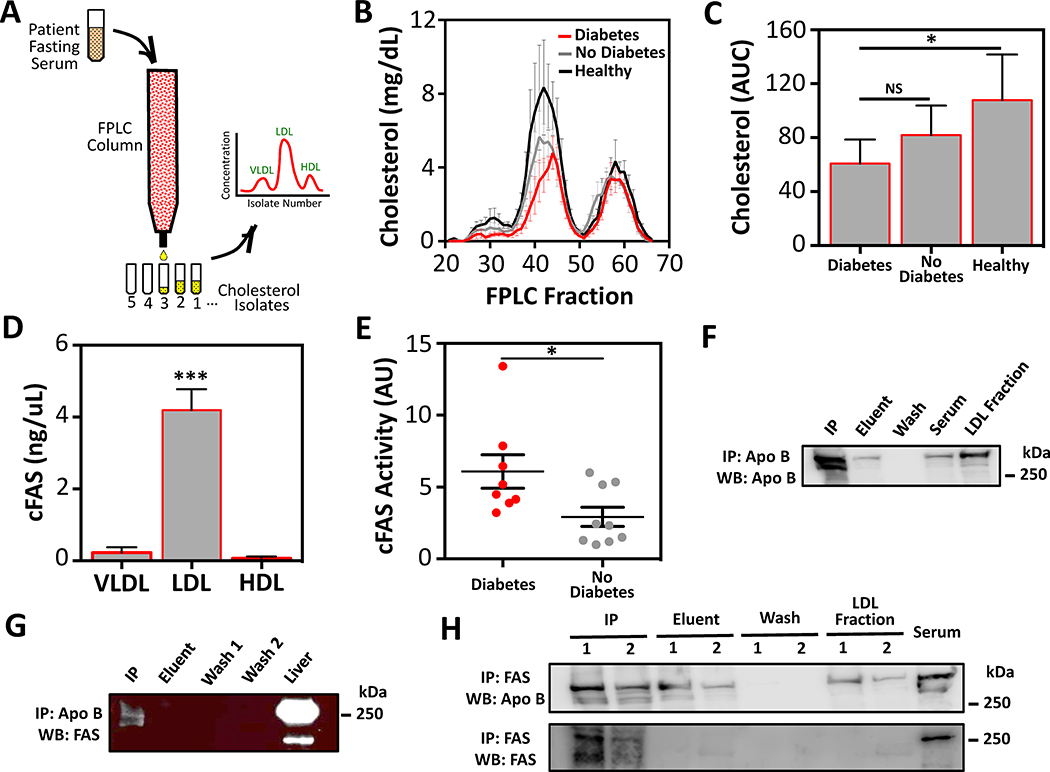
Using FPLC techniques VLDL, LDL, and HDL cholesterol fractions were isolated from fasting serum of control patients, and patients with diabetes and no diabetes (Fig. 3A). We observed that LDL cholesterol was the dominant lipoprotein in the serum of patients in all study groups (Fig. 3B). While there was no significant differences observed in VLDL, LDL, or HDL isolate fraction levels between the study patient groups (Fig. 3B), total FPLC-derived cholesterol content was observed to be lowest in patients with diabetes, and significantly less than control patients (p<0.05; Fig. 3C). This is likely because all patients with CAS were receiving moderate to high doses of Atorvastatin, Rosuvastatin, or Simvastatin (Table 1).
Figure 3:
Serum cFAS content and activity is elevated in serum LDL cholesterol isolates and co-immunoprecipitates with ApoB.
(A) Fasting serum from patients with diabetes and CAS (n=13), no diabetes and CAS (n=13), and control patients (n=13). Serum was added to an FPLC column and cholesterol isolate concentrations were evaluated to determine VLDL, LDL, and HDL concentrations. (B) Although control patients demonstrated a mild non-significant increase in serum LDL, no differences in VLDL and HDL were observed between the study groups. (C) Area under the curve (AUC) analysis demonstrated an overall increase in cholesterol isolates in control patients relative to patients with and without diabetes and CAS. *p<0.05, Kruskal-Wallis. Error bars represent the SEM. (D) cFAS content in peak VLDL, LDL, and HDL isolates was evaluated with ELISA from patients with diabetes (n=8) and patients with no diabetes (n=7). The LDL peak isolate demonstrated the highest levels of cFAS. ***p<0.001, Kruskal-Wallis. Error bars represent the SEM. (E) cFAS activity in LDL peak isolates from patients with diabetes (n=5) and patients with no diabetes (n=5) was evaluated by FAS activity assay. This demonstrated significantly higher FAS activity levels (arbitrary units; AU) in peak LDL isolates of patients with diabetes. *p<0.05, Mann-Whitney. Error bars represent the SEM. (F and G) Serum LDL fractions from a patient with diabetes was collected and ApoB immunoprecipitated. Wash, eluent, and immunoprecipitation (IP) fractions were collected. Subsequent Western blots were performed for ApoB (F) and FAS (G). An FPLC purified LDL fraction (30 μg of protein) was used as positive control for the ApoB blot, and a murine liver homogenate (10 μg of protein) was used as positive control for the FAS blot. (H) Similarly, serum LDL fractions from a patient with diabetes (1) and a patient with no diabetes (2) were collected and FAS was immunoprecipitated. The procedure wash, eluent, and IP fractions were collected and Western blots performed for ApoB (top blot), and FAS (bottom blot). Whole serum (30 μg of protein), murine liver homogenate (2.5 μg of protein) was used as positive control for ApoB and FAS blots, respectively.
cFAS was nearly exclusively found in LDL cholesterol fractions of patients with and without diabetes (p<0.001; Fig. 3D). Approximately 5% of total cFAS activity was observed in the VLDL fractions (Fig. 3D). cFAS activity in LDL cholesterol fractions from patients with diabetes was significantly higher compared to patients with no diabetes (p<0.05; Fig. 3E), and confirms that cFAS in cholesterol fractions was still biochemically active.
To determine whether cFAS interacts with serum VLDL and LDL we evaluated whether it co-immunoprecipitates with ApoB the predominant protein in these lipoproteins.[26, 27] Immunoprecipitation of ApoB from LDL cholesterol fractions demonstrated positive pulldown of cFAS (Fig. 3F&E). Conversely, immunoprecipitation of cFAS from LDL cholesterol fractions demonstrated positive pulldown of ApoB (Fig. 3H).
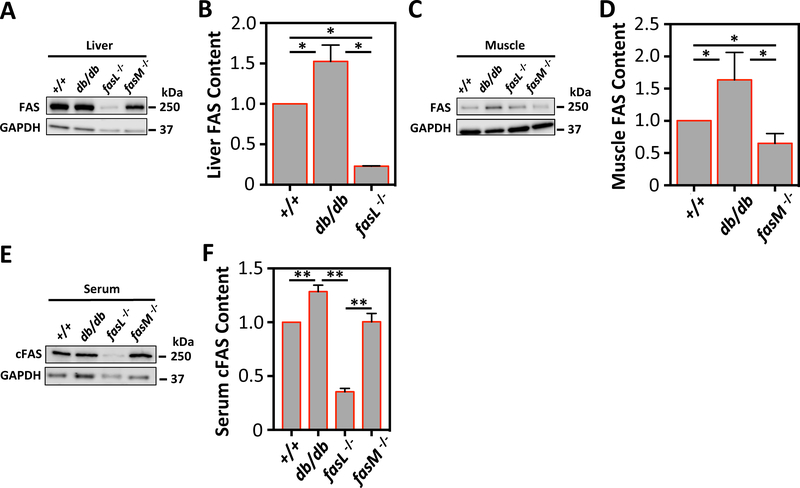
3.6. cFAS is elevated in mice with diabetes and is produced by the liver
Adult db/db mice (which have an advanced end-stage diabetes phenotype) had elevated tissue FAS in the liver and anterior tibalis skeletal muscle (p<0.05; Fig. 4A–D). Similar to patients with diabetes in our human study, db/db mice also demonstrated elevated serum cFAS (p<0.01; Fig. 4E&F). Compared to +/+ mice, mice with tissue-specific FAS knockdown in hepatocytes (fasL−/−) had significantly reduced serum cFAS (p<0.01; Fig. 4E and F), suggesting that the liver produces the majority of cFAS. On the other hand, tissue-specific FAS knockdown in skeletal muscle (fasM−/−) had no reduction in serum cFAS (Fig. 4E&F), suggesting that skeletal muscle is not a major source of cFAS.
Figure 4:
Differential expression of FAS and cFAS in murine tissue-specific liver and skeletal muscle FAS knockdowns.
(A) FAS protein content from liver homogenates of +/+, db/db, fasL−/−, and fasM−/− mice were evaluated using Western blotting. (A and B) fasL−/− mice demonstrated significantly less FAS in the liver, while db/db mice demonstrated significantly higher FAS in the liver. *p<0.05. Error bars represent the SEM. (C) FAS protein content from anterior tibialis muscle homogenates from the same mouse groups was also evaluated using Western blotting. (C and D) fasM−/− mice showed significantly less FAS in the muscle tissue, and db/db mice also showed significantly higher FAS in the muscle. *p<0.05. Error bars represent the SEM. (E) Whole serum was collected from all mouse groups, and cFAS was then evaluated using Western blot. fasL−/− mice had significantly less cFAS compared to +/+ and fasM−/−. db/db mice had significantly higher cFAS compared to all other mouse groups. **p<0.01. Error bars represent the SEM. GAPDH blot was used as loading control for all Western blots.
DISCUSSION
Diabetes is a strong, independent risk factor for stroke and cerebrovascular disease-associated mortality.[2, 5, 6] The relative risk conferred by diabetes is over and above the effect of mean arterial blood pressure, cigarette smoking, and total serum cholesterol.[1] Here we demonstrate that cFAS correlates with the presence of high-grade CAS in patients with diabetes. We also demonstrate that cFAS is present and biochemically active in non-HDL serum fractions, and likely originates from the liver. Carotid plaque isolated from patients with and without diabetes demonstrates variable FAS content, with more FAS and SFAs in the Max diseased carotid segments and in plaques isolated from patients with diabetes. These findings highlight cFAS as a potential novel and important contributor to CAS in patients with diabetes.
The patho-metabolic pathway for the markedly elevated risk of CAS in patients with diabetes is yet to be determined. Recent studies have focused on the role of wide-ranging endogenous metabolic pathways such as glycosylation of the carotid arterial wall,[28] microalbuminuria,[29] hypertriglyceridemia,[30] and low serum HDL levels.[31, 32] Serum LDL is a known contributor to diabetes-related metabolic derangements,[33] and is also an independent risk factor for the incidence of cerebrovascular disease.[34] Patients with diabetes and elevated LDL are more likely to have symptomatic carotid artery stenosis, transient ischemic attacks, and resultant strokes.[35] However, several recent studies have proposed that LDL content alone does not fully explain the higher risk of stroke in patients with diabetes who also receive statin and other cholesterol lower therapy.[36, 37] A recent meta-analysis of over 18,000 patients with diabetes demonstrated decreased stroke events in patients receiving cholesterol-lower therapy. However, among patients receiving cholesterol-lower therapy, patients with diabetes still had a 39% relative increase in stroke events compared to patients with no diabetes.[36] Recent reports also argue that ApoB, the predominant protein in non-HDL cholesterol, is a more important predictor of cardiovascular disease.[38, 39] Thus, it is currently unclear whether specific serum molecular factors can interact with ApoB and/or non-HDL cholesterol to ultimately influence atheroprogression in the peripheral arterial system.
We observed a significant positive correlation between cFAS activity and serum glucose. This finding is consistent with our observation that cFAS activity is elevated in the serum and LDL cholesterol fractions of patients with diabetes, and may be a reflection of the extent of insulin resistance in our study patients.[17] Previously, hyperinsulinemia as a result of insulin resistance in the setting of diabetes was observed to be an important FAS effector. Not only does insulin increase the rate of fasn gene transcription,[12, 40] but it also increases FAS enzyme activity as observed in our study in patients with diabetes. Insulin-induced FAS overexpression also leads to downstream transcription factor activation,[40] altered cellular metabolism, and modified cell membrane composition in the liver, skeletal muscle, and adipose tissue.[41] Recently one study demonstrated that deletion of FAS in macrophages prevented diet-induced insulin resistance and membrane retention of cholesterol.[42] These findings highlight that the response to insulin and metabolic stress in the setting of diabetes can affect FAS activity and cholesterol homeostasis.
Previous work demonstrates that FAS is glycosylated, and this is essential for its allosteric confirmations necessary for its catalytic domains.[43] This could help explain why unlike cFAS activity, cFAS content did not correlate with serum glucose. Similarly, although we observed that cFAS activity was higher in the serum of patients with diabetes compared to patients without diabetes, we did not observe a similar difference in the cFAS content between these groups (data not shown). Our findings suggest that although patients with CAS have an overall higher level of serum cFAS (Fig. 1A), patients with diabetes who are more prone to have elevated serum glucose levels are more likely to have active serum cFAS, which can lead to downstream consequences.
The role of FAS in atheroprogression has never been previously explored. In this study, we provide the first evidence that implicates FAS in both the plaque tissue and serum of patients with high-grade CAS. The incremental difference in cFAS activity in patients with diabetes and CAS suggests that cFAS is a marker of advanced atherosclerotic disease in patients who are typically at higher risk for cerebrovascular complications. Additionally, differential FAS content in variably diseased carotid plaque suggests that FAS localizes to areas of higher carotid disease burden. Presumably, local factors within the arterial wall can influence FAS expression and content within the plaque. Although we observed clear differences in the content of FAS and SFAs in Max diseased plaque portions and in patients with diabetes (Fig. 2J–M), we observed no difference in carotid plaque fasn expression in patients with and without diabetes (Fig. 2H). One potential explanation for this is that VLDL lipoproteins derived from the liver that are subsequently converted to LDL in the intravascular space, may serve as carriers for cFAS cargo that is ultimately delivered to the peripheral arterial wall (Fig. 5]). Increased active cFAS in the vessel wall can in turn lead to further accumulation of SFAs, that can lead to plaque inflammation, lesion progression and instability.[44]
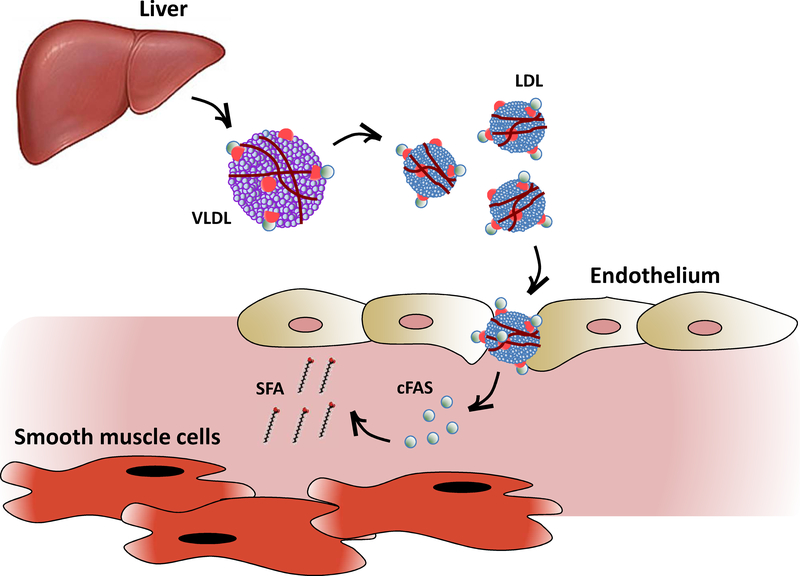
Figure 5:
cFAS originates from the liver and may be transported by LDL to the peripheral vasculature to influence plaque formation.
cFAS produced by the liver is extruded into the blood stream in semi-soluble form conjugated to ApoB in non-HDL cholesterol particles such as VLDL and LDL. Intravascular LDL particles traverse the endothelium along with their cFAS cargo, which is then delivered to the sub-endothelium. Accumulation of cFAS in the sub-endothelium is thought to contribute to SFA plaque content and peripheral arterial atheroprogression.
Our study establishes a novel link between cFAS and LDL, and demonstrates a unique biochemical interaction between cFAS and ApoB. Previous studies provided a link between FAS and lipoproteins produced by the liver.[43] For example, in human subjects who consumed diets high in carbohydrates (10% of calories as fat and 75% as carbohydrates) hepatic FAS was found to be responsible for the synthesis of the majority of circulating VLDL triglycerides.[45] Interestingly, the lipoprotein contributions from hepatic FAS were not as high in patients who consumed high fat and carbohydrate Western diets.[18] This unexpected finding may have underestimated the role of FAS, since lipoprotein contributions from extra-hepatic FAS and cFAS was not evaluated. The content, activity, and contributions of cFAS in diet-influenced lipoprotein production are currently unknown. Similarly, the contribution of cFAS to lipoprotein content in patients with diabetes with variable degrees of metabolic stress and peripheral atheroprogression is yet to be explored and is certainly a ripe area for future investigation.
We observed a marked decreased in serum cFAS in fasL−/− mice. This highlights that the liver produces the bulk of cFAS, while no change in the level of cFAS in fasM−/− mice demonstrates that skeletal muscle is not a major source of cFAS. These findings are consistent with prior findings that in the fed state hepatic FAS can synthesize lipids that are either stored as lipid droplets and/or secreted in VLDL.[43] In both human subjects with non-alcoholic fatty liver disease and ob/ob mice, increased hepatic FAS activity is thought to be an important contributor to the development of fatty liver.[27] Additionally, genetic alteration of FAS expression in the liver imposes significant metabolic consequences to the host.[23] Therefore, the contributions of the liver to cFAS are logical and would also be consistent with the proposed model of cFAS trafficking from the liver to the peripheral vasculature via lipoprotein particles also synthesized by the liver.
We acknowledge that our study has some limitations. First, the limited number of patients evaluated here can lead to sampling error and not fully account for the range of other pathologies that affect patients with CAS. Future studies will aim to enroll larger patient groups for further validation and confirmation across various patient demographics. Second, with the limited number of patients in each group we were not able to fully match for age and gender demographics particularly in the control group. It is possible that age and gender could have confounded our data in the control patient group, although we observed that age and gender in CAS patients did not have a significant effect on cFAS (Supplemental Fig. 1C&D). Third, FPLC techniques do not differentiate between LDL and LDL-like particles (such as Lipoprotein(a)). It is possible that cFAS differentially associates with such particles, and further fractionation of FPLC-derived LDL isolates may help determine this. Furthermore, we acknowledge that the role of cFAS in atheroprogression cannot be proven except with a prospective modulation of the enzyme. Immediate next steps to evaluate this are focusing on the rate of atheroprogression in fasL−/− and db/db mice treated with selective cFAS inhibitors. These studies are anticipated to determine whether cFAS indeed plays an essential role in atheroprogression and plaque instability.
In conclusion, we are the first to report that cFAS is present in high levels in patients with CAS, and enzyme activity is particularly higher in patients with diabetes. We also observed that cFAS correlates with serum glucose, interacts with LDL cholesterol particles, and correlates with carotid plaque FAS. We postulate that VLDL and LDL are carriers of cFAS cargo to the peripheral carotid tissue of patients with diabetes leading to increased risk of atheroprogression.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Washington University School of Medicine Vascular Surgery Biobank Core Facility. The authors thank Mrs. Amanda Penrose for her assistance with collection, preparation, and processing of Biobank Core Facility research samples and their analysis.
FINANCIAL SUPPORT
This work was supported by grants from the Vascular Cures Foundation Wylie Scholar Award (MAZ), American Surgical Association Research Fellowship Award (MAZ), Society for Vascular Surgery Foundation Research Investigator Award (MAZ), Washington University School of Medicine Diabetes Research Center NIH/NIDDK P30 DK020589 (MAZ), NIH/NHLBI K08 HL132060 (MAZ), and NIH/NIDDK R01 DK101392 (CFS).
Footnotes
CONFLICT OF INTEREST
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ergul A, et al. , Cerebrovascular complications of diabetes: focus on stroke. Endocr Metab Immune Disord Drug Targets, 2012. 12(2): p. 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeerakathil T, et al. , Short-term risk for stroke is doubled in persons with newly treated type 2 diabetes compared with persons without diabetes: a population-based cohort study. Stroke, 2007. 38(6): p. 1739–43. [DOI] [PubMed] [Google Scholar]
- 3.Kuebler TW, et al. , Diabetes mellitus and cerebrovascular disease: prevalence of carotid artery occlusive disease and associated risk factors in 482 adult diabetic patients. Diabetes Care, 1983. 6(3): p. 274–8. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M, et al. , The NASCET-ACAS plaque project. North American Symptomatic Carotid Endarterectomy Trial. Asymptomatic Carotid Atherosclerosis Study. Stroke, 1993. 24(12 Suppl): p. I24–5; discussion I31–2. [PubMed] [Google Scholar]
- 5.Gregg EW, et al. , Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med, 2014. 370(16): p. 1514–23. [DOI] [PubMed] [Google Scholar]
- 6.Laing SP, et al. , Mortality from cerebrovascular disease in a cohort of 23 000 patients with insulin-treated diabetes. Stroke, 2003. 34(2): p. 418–21. [DOI] [PubMed] [Google Scholar]
- 7.Edsfeldt A, et al. , Sphingolipids Contribute to Human Atherosclerotic Plaque Inflammation. Arterioscler Thromb Vasc Biol, 2016. 36(6): p. 1132–40. [DOI] [PubMed] [Google Scholar]
- 8.Pasterkamp G, Methods of accelerated atherosclerosis in diabetic patients. Heart, 2013. 99(10): p. 743–9. [DOI] [PubMed] [Google Scholar]
- 9.Semenkovich CF, Regulation of fatty acid synthase (FAS). Prog Lipid Res, 1997. 36(1): p. 43–53. [DOI] [PubMed] [Google Scholar]
- 10.Smith S, The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J, 1994. 8(15): p. 1248–59. [PubMed] [Google Scholar]
- 11.Claycombe KJ, et al. , Insulin increases fatty acid synthase gene transcription in human adipocytes. Am J Physiol, 1998. 274(5 Pt 2): p. R1253–9. [DOI] [PubMed] [Google Scholar]
- 12.Paulauskis JD and Sul HS, Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J Biol Chem, 1989. 264(1): p. 574–7. [PubMed] [Google Scholar]
- 13.Wu M, et al. , Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc Natl Acad Sci U S A, 2011. 108(13): p. 5378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menendez JA, et al. , Fatty acid synthase: association with insulin resistance, type 2 diabetes, and cancer. Clin Chem, 2009. 55(3): p. 425–38. [DOI] [PubMed] [Google Scholar]
- 15.Menendez JA and Lupu R, Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer, 2007. 7(10): p. 763–77. [DOI] [PubMed] [Google Scholar]
- 16.Wang YY, et al. , Fatty acid synthase as a tumor marker: its extracellular expression in human breast cancer. J Exp Ther Oncol, 2004. 4(2): p. 101–10. [PubMed] [Google Scholar]
- 17.Fernandez-Real JM, et al. , Extracellular fatty acid synthase: a possible surrogate biomarker of insulin resistance. Diabetes, 2010. 59(6): p. 1506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudgins LC, et al. , Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res, 2000. 41(4): p. 595–604. [PubMed] [Google Scholar]
- 19.Chakravarthy MV, et al. , “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab, 2005. 1(5): p. 309–22. [DOI] [PubMed] [Google Scholar]
- 20.Funai K, et al. , Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J Clin Invest, 2013. 123(3): p. 1229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullman AH and White HB 3rd, Assay of fatty acid synthase using a bicyclic dione as substrate. Methods Enzymol, 1981. 72: p. 303–6. [DOI] [PubMed] [Google Scholar]
- 22.Zayed MA, et al. , Diabetes adversely affects phospholipid profiles in human carotid artery endarterectomy plaques. J Lipid Res, 2018. 59(4): p. 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakravarthy MV, et al. , Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell, 2009. 138(3): p. 476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaid M, et al. , Associations of serum LDL particle concentration with carotid intimamedia thickness and coronary artery calcification. J Clin Lipidol, 2016. 10(5): p. 11951202 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitagawa K, et al. , Cumulative Effects of LDL Cholesterol and CRP Levels on Recurrent Stroke and TIA. J Atheroscler Thromb, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sniderman AD, et al. , Calculation of LDL apoB. Atherosclerosis, 2014. 234(2): p. 373–6. [DOI] [PubMed] [Google Scholar]
- 27.Wiegman CH, et al. , Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes, 2003. 52(5): p. 1081–9. [DOI] [PubMed] [Google Scholar]
- 28.Miyazawa T, et al. , Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids, 2012. 42(4): p. 1163–70. [DOI] [PubMed] [Google Scholar]
- 29.Mykkanen L, et al. , Microalbuminuria and carotid artery intima-media thickness in nondiabetic and NIDDM subjects. The Insulin Resistance Atherosclerosis Study (IRAS). Stroke, 1997. 28(9): p. 1710–6. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad J, et al. , Postprandial hypertriglyceridemia and carotid intima-media thickness in north Indian type 2 diabetic subjects. Diabetes Res Clin Pract, 2005. 69(2): p. 142–50. [DOI] [PubMed] [Google Scholar]
- 31.Generoso G, et al. , Diabetes alters the association between high-density lipoprotein subfractions and carotid intima-media thickness: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Diab Vasc Dis Res, 2018. 15(6): p. 541–547. [DOI] [PubMed] [Google Scholar]
- 32.Tan HC, et al. , Relationships between cholesterol efflux and high-density lipoprotein particles in patients with type 2 diabetes mellitus. J Clin Lipidol, 2011. 5(6): p. 467–73. [DOI] [PubMed] [Google Scholar]
- 33.Ray KK, et al. , The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: the good the bad and the uncertain: a comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011. Eur Heart J, 2014. 35(15): p. 960–8. [DOI] [PubMed] [Google Scholar]
- 34.Touboul PJ, et al. , Mannheim carotid intima-media thickness and plaque consensus (2004–2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis, 2012. 34(4): p. 290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kissela BM, et al. , Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care, 2005. 28(2): p. 3559. [DOI] [PubMed] [Google Scholar]
- 36.Cholesterol Treatment Trialists C, et al. , Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet, 2008. 371(9607): p. 117–25. [DOI] [PubMed] [Google Scholar]
- 37.Schofield JD, et al. , Diabetes Dyslipidemia. Diabetes Ther, 2016. 7(2): p. 203–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walldius G, Apolipoprotein B (apoB) more closely related to subclinical atherosclerosis than non-HDL cholesterol and LDL cholesterol. J Intern Med, 2010. 268(6): p. 549–51. [DOI] [PubMed] [Google Scholar]
- 39.Zhang HW, et al. , ApoB is superior to LDL-C or non-HDL-C as a lipid marker for predicting the presence and severity of atherosclerosis in female patients with myocardial infarction. Hellenic J Cardiol, 2017. 58(3): p. 223–225. [DOI] [PubMed] [Google Scholar]
- 40.Griffin MJ and Sul HS, Insulin regulation of fatty acid synthase gene transcription: roles of USF and SREBP-1c. IUBMB Life, 2004. 56(10): p. 595–600. [DOI] [PubMed] [Google Scholar]
- 41.Semenkovich CF, Coleman T, and Fiedorek FT Jr., Human fatty acid synthase mRNA: tissue distribution, genetic mapping, and kinetics of decay after glucose deprivation. J Lipid Res, 1995. 36(7): p. 1507–21. [PubMed] [Google Scholar]
- 42.Wei X, et al. , Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature, 2016. 539(7628): p. 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen-Urstad AP and Semenkovich CF, Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta, 2012. 1821(5): p. 74753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sudheendran S, Chang CC, and Deckelbaum RJ, N-3 vs. saturated fatty acids: effects on the arterial wall. Prostaglandins Leukot Essent Fatty Acids, 2010. 82(4–6): p. 205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hudgins LC, et al. , Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest, 1996. 97(9): p. 2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang JS, et al. , Natural fatty acid synthase inhibitors as potent therapeutic agents for cancers: A review. Pharm Biol, 2016. 54(9): p. 1919–25. [DOI] [PubMed] [Google Scholar]
- 47.Loftus TM, et al. , Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science, 2000. 288(5475): p. 2379–81. [DOI] [PubMed] [Google Scholar]
- 48.Wei X, et al. , De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. J Biol Chem, 2011. 286(4): p. 2933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.