Abstract
Objective
Abdominal obesity and wall thickness of the central arteries have been associated with higher risk of cardiovascular disease (CVD). Despite the higher burden of overweight and CVD disease among African-Americans, limited data are available on the association of abdominal obesity with aortic wall thickness in African-Americans. We assessed the cross-sectional and the longitudinal associations of abdominal obesity with aortic intima-media thickness (aIMT) in a cohort of African-Americans from the Jackson Heart Study (JHS).
Methods
Data on aIMT and repeated-measures of waist circumference (WC) and waist-to-height ratio (WHtR) from 1,572 participants, and on abdominal subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), and aIMT from 1,223 participants, were analyzed. aIMT was measured at proximal ascending aorta (PA-aIMT), proximal descending aorta (PD-aIMT), and distal aorta (bifurcation) using cardiac magnetic resonance. SAT and VAT were measured using computerized tomography.
Results
WC and WHtR are longitudinally associated with PA-aIMT and PD-aIMT; SAT and VAT are associated with PA-aIMT only. Only WC is associated with distal aIMT.
Conclusions
Abdominal obesity measures are associated with increased proximal aIMT in adult African-Americans. Only WC is associated with wall thickness in all the three segments of the aorta.
Keywords: Abdominal adiposity, Obesity, Subcutaneous Adipose Tissue, Visceral fat, African America
INTRODUCTION
Cardiovascular disease (CVD) is the largest contributor of cause of death globally, with an estimated 17 million premature deaths due to CVD in 2015.1 In the United States, 11.5% of adults are diagnosed with heart disease, with one in four deaths being attributable to heart disease.2 Changes in arterial structure predicts CVD morbidity and mortality and is associated with other CVD risk factors.3 Carotid artery intima-media thickness (cIMT) has been shown to be a reliable indicator of clinical atherosclerosis and was associated with incident and prevalent CVD and future vascular events.4–6 While cIMT is easier to measure, aortic wall thickness should also be investigated because it has higher specificity and positive predictive value for predicting coronary artery disease.7, 8 Additionally, aortic wall thickness is associated with short- and long- term CVD morbidity and mortality9, 10, even in the absence of detectable atherosclerosis.11, 12
Abdominal obesity—manifested by increased waist circumference (WC)—has also been associated with increased CVD morbidity and mortality.13, 14 Abdominal fat is partitioned into two major compartments: subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT); both have been found to be associated with CVD risk.15–17
In the US, African-Americans have an increased risk of CVD morbidity and mortality, such as stroke and heart failure, and experience the highest mortality rate of coronary heart disease (CHD) of any ethnic group.18–20 Perhaps because they not only suffer from a higher prevalence of CVD risk factors, such as overweight and hypertension, but also they are affected by these risk factors at a younger age.21, 22 Moreover, some evidence suggests that African-Americans have higher cIMT compared to whites.3 However, data on the association of abdominal obesity with aortic thickness among African-Americans are scarce, despite their disproportionate burden of CVD and its risk factors.
In this study, we assessed and compared markers of abdominal obesity: WC, waist/height ratio (WHtR), SAT, and VAT in relation to aortic intima-media thickness (aIMT) in three segments of the aorta: proximal ascending arch (PA-aIMT), proximal descending arch (PD-aIMT), and distal (bifurcation) aorta, in a large sample of African-Americans from the Jackson Heart Study (JHS)
MATERIALS AND METHODS
Study Population
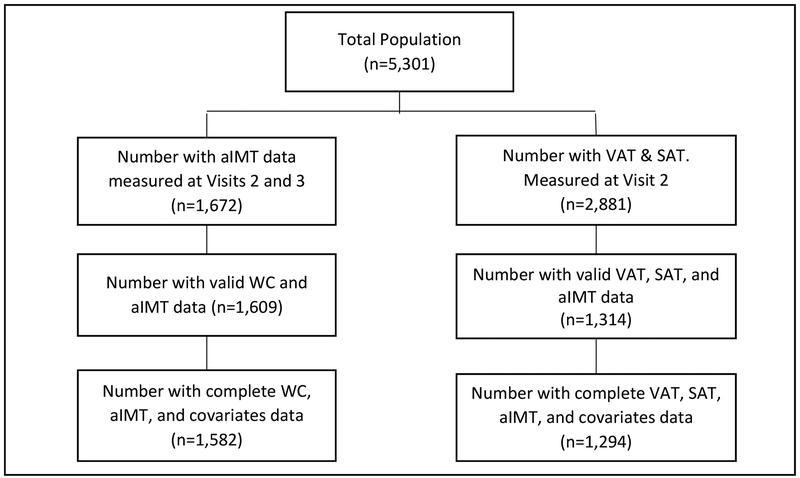
The JHS is a large, prospective community-based cohort of 5,301 African-Americans 21 to 84 years of age who were recruited from the tri-county area surrounding Jackson, Mississippi.23 Three data collection rounds were performed: Visit 1 (baseline, 2000–2004); Visit 2 (2005–2008), and Visit 3 (2009–2013). Participants eligible for the present study are those who have WC and covariates data from all the three visits, and who also completed both the abdominal computed tomography (CT, n=2,881) and the cardiac magnetic resonance (CMR, n=1,672) ancillary studies that were performed during Visits 2 and 3, respectively. This resulted in a sample of 1,582 participants with complete data on WC, aIMT and covariates, and 1,294 participants with complete data on VAT or SAT, aIMT, and covariates (Figure 1).
Figure 1:
Flowchart of the study sample selection.
aIMT = aortic intima-media thickness, SAT = subcutaneous adipose tissue, VAT = Visceral adipose tissue, WC = waist circumference.
The JHS was approved by the Institutional Review Boards of Jackson State University, Tougaloo College, and the University of Mississippi Medical Center in Jackson, Mississippi. All study participants gave written informed consent.
Outcome Measurements
CMR was performed once during Visits 2 or 3 using a large-bore (70-cm) 1.5T Siemens Espree scanner (Erlangen, Germany) with TIM cardiac software and multichannel matrix surface coil.24 Aortic wall thickness was measured on ECG-gated, axial black-blood fast-spin echo sequence images as described elsewhere.25 Mean wall thickness (mm) was measured using custom Matlab software at Wake Forest School of Medicine. aIMT data at proximal ascending, proximal descending, and distal aorta (2cm above the aortic bifurcation) were used for this study.
Exposure Measurements
WC was recorded at each visit as the average of two measurements taken at the level of the umbilicus. WHtR was calculated by dividing waist circumference by height in centimeters. VAT and SAT were measured during once during Visit 2 using computerized tomography (CT). Imaging of the abdomen was conducted in the supine position by multi-detector CT scanning (GE Healthcare Light-speed 16 Pro, Milwaukee, WI) as previously described.26, 27 Briefly, a series of continuous scout images through the lower abdomen from L3 to S1 were used to assess abdominal adipose tissue depots using the lumbosacral junction (centered at L4–L5) as an anatomic landmark. Twenty-four, 2-mm thick slices (48 mm total coverage) were acquired and analyzed. The abdominal muscular wall was manually traced, and the VAT and SAT volumes were measured by a semiautomatic segmentation technique using GE Advantage Windows software (Advantage Windows, GE Healthcare, Waukesha, WI). VAT and SAT volumes were the sum of voxels over the 24 slices that fell into the tissue attenuation range of −190 to −30 Hounsfield units.26, 27 Inter-reader (two readers) reproducibility was assessed on a subset of 60 randomly selected scans. The interclass correlations were >0.95 for both SAT and VAT measurements.
Covariates
Demographic, behavioral, and socio-economic indicators were self-reported via a standard questionnaire and were collected at each visit. At each examination, systolic and diastolic blood pressures were measured in the right arm using the random-0 blood pressure sphygmomanometer (Hawksley and Sons Limited, Sussex, United Kingdom). Two measurements were taken after five minutes resting in a seated position, with the second blood pressure taken after waiting an additional minute. The average of the two measurements was used. Fasting plasma glucose (FPG) and blood lipids were assessed using standard laboratory techniques after 12 hours of fasting. Mean arterial pressure (MAP) was calculated from the formula [(2 × diastolic BP) + systolic BP)]/3. Data missingness was negligible in this study and ranged from 1% for WC to 1.7% for WHtR for all three follow up visits. With the exception of income, for which data were complete on 85.1% of all participants, all the covariate had ≥92% complete data.
Statistical Analyses
Descriptive statistics were computed using frequencies for categorical data and means for continuous data. Bivariate linear regression was used to assess the crude associations of abdominal obesity variables at baseline with PA-aIMT, PD-aIMT, and distal aIMT. Multivariable linear regression was used to assess the adjusted associations of VAT and SAT with aIMT. Separate models were run for each aortic segment. All models were adjusted for age, sex, LDL cholesterol, HDL cholesterol, triglycerides, FPG, MAP, as well as smoking status, income, and daily alcohol consumption. Because aIMT measurements were performed during the 2nd and 3rd visits temporal order can be established only between baseline covariates and the outcomes. Therefore, only baseline covariates were included in the regression models. Next, generalized linear mixed (GLM) models were analyzed to assess the longitudinal associations of WC and WHtR, over time, with aIMT. WC or WHtR, as well as age, LDL cholesterol, HDL cholesterol, triglycerides, FPG, and MAP were included in the models as repeated-measures main effects. These models allowed for the assessment of the effect of the change over time of WC, or WHtR, on aIMT while controlling for the change of the other covariates. Additionally, ID number was included in the statistical model as a random-effect variable to account for the individual differences between the participants. Several correlation matrices were tested for the mixed models (e.g., scaled identity, diagonal, and autoregressive,) with scaled identity consistently providing the best fit of the data, so it was used in the analysis. Non-significant variables were excluded from the models using reiterative approach for GLM analyses, and backward elimination method for linear regression models. However, markers of abdominal obesity were forced in the models regardless of statistical significance. BMI was not included in the analyses because the study aims to examine the effect of abdominal obesity on aIMT. To check for the linearity of the effect with age, age2 was tested in all the models. However, based on Akaike’s Information Criterion for the mixed models and R2 for the linear regression models, the models’ fit either improved slightly or worsened slightly. Therefore, the results of the linear models are reported. Repeating the analyses after excluding participants with baseline history of CVD only strengthened the studied associations slightly. Therefore, the results of the whole sample are reported. We also tested the interactions of gender with measures of abdominal obesity (e.g., sex × WC) on all outcomes, but none was significant. SPSS (V. 24) statistical software (IBM, Armonk, NY) was used in the analysis, with alpha set to 0.05.
RESULTS
The sample is mainly of middle-age African-Americans (63.5% women), with an average follow time of 8.7 years. Both WC and WHtR increased between the 1st and the 3rd visits (in men WC increased by 3.2 cm and WHtR by 0.02 units; in women WC increased by 2.7 cm and WHtR by 0.02 units, p<0.01 for all). Men and women have the same WC on average, but the relative contribution of VAT to abdominal obesity is larger in men. Men are slightly leaner (WHtR), and they smoke nearly twice as much as women. Table 1 describes important characteristics of the study sample at baseline, by sex.
Table 1.
Descriptive statistics of the study population (mean ± SD), by sex
| Characteristic (mean ± SD) | Men (37.5%) | Women (63.5%) | P |
|---|---|---|---|
| Age (year)† | 54. 6 ± 13.0 | 55.8 ± 12.7 | 0.001 |
| WC (cm)† | 101.3 ± 15.1 | 100.4 ± 16.7 | 0.049 |
| WHtR† | 0.57 ± 0.08 | 0.61 ± 0.10 | <0.001 |
| LDL-cholesterol (mg/dL)† | 129.4 ± 36.7 | 126.1 ± 26.1 | 0.005 |
| HDL-cholesterol (mg/dL)† | 45.9 ± 12.4 | 55.2 ± 14.5 | <0.001 |
| Triglycerides (mg/dL)† | 116.3 ± 100.5 | 100.0 ± 65.3 | <0.001 |
| FPG(mg/dL)† | 99.4 ± 31.3 | 98.7 ± 31.9 | 0.477 |
| MAP (mmHg)† | 96.8 ± 11.1 | 93.6 ± 11.2 | <0.001 |
| Alcohol drinks (/week)† | 5.7 ± 9.6 | 1.7 ± 4.9 | <0.001 |
| Current Smoking (%)† | 18.3% | 10.3% | <0.001 |
| SAT (cm3)‡ | 1722.2 ± 806.6 | 2657.5 ± 962.9 | <0.001 |
| VAT (cm3)‡ | 883.0 ± 415.1 | 802.3 ± 362.5 | <0.001 |
| Mean PA-alMT (mm)§ | 3.10 ± 0.56 | 3.06 ± 0.57 | 0.253 |
| Mean PD-alMT (mm)§ | 3.06 ± 0.61 | 3.05 ± 0.60 | 0.844 |
| Mean distal-alMT (mm)§ | 2.44 ± 0.67 | 2.31 ± 0.67 | <0.001 |
FPG = fasting plasma glucose; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MAP = mean arterial pressure; PA-aIMT = proximal ascending aortic intima-media thickness; PD-aIMT = proximal descending aortic intima-media thickness; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue; WC = waist circumference; WHtR = waist-to-height ratio.
denotes baseline values.
measured during Visit 2.
measured during Visits 2 or 3. Mann-Whitney test was used for all comparisons of continuous variables and chi-square test was used for comparing smoking status.
Table 2 shows the crude associations of baseline markers of abdominal obesity with PA-, PD-, and distal-aIMT. WC is associated with aIMT at all the three locations, while WHtR is associated with proximal, but not distal, aIMT. SAT and VAT are associated with PA-aIMT, but not PD-aIMT. Interestingly, both SAT and VAT have an inverse correlation with distal (bifurcation) aIMT, but only SAT is statistically significant.
Table 2.
Crude associations of measures of abdominal obesity with proximal ascending-, proximal descending-, and distal aIMT.
| Variable | PA-aIMT | PD-aIMT | Distal aorta | |||
|---|---|---|---|---|---|---|
| B | P | B | P | B | P | |
| WC (cm)† | 0.29 | <0.001 | 0.20 | <0.001 | 0.07 | 0.005 |
| WHtR† | 0.27 | <0.001 | 0.18 | <0.001 | 0.01 | NS |
| SAT (cm3) | 0.09 | 0.001 | 0.04 | NS | −0.06 | 0.043 |
| VAT (cm3) | 0.10 | 0.001 | 0.02 | NS | −0.04 | NS |
B = standardized coefficient; PA-aIMT = proximal ascending aortic intima-media thickness; PD-aIMT = proximal descending aortic intima media thickness; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue; WC = waist circumference, WHtR = waist-to-height ratio.
denotes baseline values.
Table 3 shows the adjusted associations of markers of abdominal obesity with aIMT at the three locations. All measures of abdominal obesity are associated with PA-aIMT. WC, WHtR and SAT (borderline significance) are associated with PD-aIMT. None of the markers of abdominal obesity is associated with distal aIMT except for WC, which also shows borderline significance. HDL cholesterol is inversely associated with aIMT of both proximal segments, but not with distal aorta, independent from all tested measures of abdominal obesity. Age is independently associated with PD-aIMT only. MAP and triglycerides (inversely) are associated with PD-aIMT independent of WC, but not from SAT and VAT. Finally, current smoking is associated with PD-aIMT and distal aIMT with a trend of a stronger effect on distal aorta.
Table 3.
Adjusted associations of markers of abdominal obesity with proximal ascending-, proximal descending-, and distal aIMT.
| Variable | PA-aIMT | PD-aIMT | Distal aorta | |||
|---|---|---|---|---|---|---|
| B | P | B | P | B | P | |
| n = l,519 | n = 1,543 | n = 1,496 | ||||
| WC (cm)† | 0.27 | <0.001 | 0.17 | <0.001 | 0.06 | 0.040 |
| Sex | - | NS | - | NS | −0.13 | <0.001 |
| Age (year) | - | NS | 0.15 | <0.001 | - | NS |
| MAP (mmHg) | - | NS | 0.06 | 0.033 | - | NS |
| Triglycerides (mg/dL) | −0.07 | 0.005 | −0.08 | 0.005 | - | NS |
| HDL-C (mg/dL) | −0.07 | 0.008 | −0.07 | 0.008 | - | NS |
| Current smoking | - | NS | 0.05 | 0.020 | 0.10 | <0.001 |
| n = 1,519 | n = 1,543 | n = 1,493 | ||||
| WHtR† | 1.05 | <0.001 | 0.14 | <0.001 | 0.03 | 0.236 |
| Sex | −0.10 | <0.001 | - | NS | −0.12 | <0.001 |
| Age (year) | - | NS | 0.15 | <0.001 | - | NS |
| MAP (mmHg) | - | NS | 0.07 | 0.012 | - | NS |
| Triglycerides (mg/dL) | −0.07 | 0.004 | −0.08 | 0.002 | - | NS |
| HDL-C (mg/dL) | −0.06 | 0.020 | −0.09 | 0.001 | - | NS |
| Current smoking | - | NS | 0.14 | 0.028 | 0.10 | <0.001 |
| n = 1,161 | n = 1,200 | n = 1155 | ||||
| SAT (per 10 cm3) | 1.33 | <0.001 | 0.54 | 0.060 | −0.40 | 0.227 |
| Sex | −0.99 | 0.005 | - | NS | - | NS |
| Age (year) | - | NS | 1.00 | <0.001 | - | NS |
| HDL-C (mg/dL) | −0.70 | 0.025 | −0.93 | 0.005 | −1.07 | 0.001 |
| Current smoking (yes) | - | NS | - | NS | - | NS |
| n = 1,161 | n = 1,200 | n = 1155 | ||||
| VAT (per 10 cm3) | 0.65 | 0.030 | -0.26 | 0.432 | −0.51 | 0.100 |
| Sex | - | NS | - | NS | −0.64 | 0.041 |
| Age (year) | - | NS | 1.07 | <0.001 | - | NS |
| HDL-C (mg/dL) | −0.82 | 0.007 | −0.97 | 0.002 | −1.13 | <0.001 |
| Current smoking (yes) | - | NS | - | NS | 0.49 | 0.096 |
All models are adjusted for age, sex, current smoking status, income, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, fasting plasma glucose, and mean arterial pressure (MAP). Variables that are not significant in any model are not included in the table. PA-aIMT = proximal ascending aortic intima-media thickness; PD-aIMT = proximal descending aortic intima media thickness; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue; WC = waist circumference; WHtR = waist-to-height ratio.
Sex (0 = male, 1 = female). B = standardized coefficient.
() = denotes repeated-measure (mixed models) analysis.
Because sex is independently associated with PA-aIMT in WHtR and SAT models, and with distal aIMT in all models except for SAT (Table 3), we reran these models separately for men and women. For PA-aIMT, WHtR is significant in both men and women with a stronger effect in women (B = 0.10; p = 0.013, and B = 0.21; p < 0.001, in men and women, respectively,) while SAT is significant in women only (B = 0.17; p < 0.001). For distal aIMT, WC and WHtR are not associated with distal aIMT in either sex, while VAT is associated with distal aIMT only in women (B = −0.07; p = 0.034). All results are presented in standardized coefficients to enable comparison of the effect sizes of the independent variables on aIMT.
DISCUSSION
In this cohort of adult African-Americans WC and WHtR are longitudinally associated with both proximal ascending and proximal descending aIMT after adjusting for potential confounders. SAT and VAT are associated only with proximal aIMT. The effect sizes of these factors are of similar magnitude to those reported in other studies (Supplementary Tables 1–4 show the unstandardized coefficients of markers of abdominal obesity with PA-aIMT).28, 29 On the other hand, none of the markers of abdominal obesity is associated with distal aIMT. Contrary to the accumulating evidence indicating a stronger effect of VAT than SAT or WC on CVD risk,29–31 we found the effect size of VAT on aIMT of both proximal aortic segments the weakest among the four measures, and it was not associated with distal aIMT. This discrepancy could be due to the previously published data coming from mostly Caucasians. African-Americans and Caucasians have different body fat distributions, where the former have proportionately less VAT and more SAT than the latter.32, 33
That WC has a stronger association with aIMT than SAT or VAT underscores its potential public health utility in identifying high-risk groups for CVD among African-Americans—not only because of the easiness and simplicity of measuring WC, but also because proximal aIMT has been shown to accurately predict CVD morbidity and mortality, even in the absence of detectable atherosclerotic disease.7, 12 Furthermore, the evidence show that WC is a better predictor of CVD morbidity and mortality than BMI.34
Since aortic wall thickness is closely associated with blood pressure, an important CVD risk factor, we assessed the association of measurements of aIMT with systolic and diastolic blood pressure in order to evaluate the potential impact of these findings on CVD risk. aIMT at all three locations is positively correlated with both systolic and diastolic blood pressures, but the association was statistically significant only with PD-aIMT. For each 1 mm increase in PD-aIMT thickness systolic blood pressure increased by 3.2 mmHg and diastolic blood pressure increased by 2.5 mmHg, after adjusting for age, sex and blood lipids (data not shown). However, it is hard to gauge the clinical implications of these findings because aortic wall thickness is not the only mechanism by which obesity can affect CVD risk.
Notably, HDL cholesterol is inversely associated with both proximal and distal aIMT, independent of all measures of abdominal obesity in this sample. This finding agrees with other reports of an inverse association of HDL cholesterol with arterial wall thickness.35, 36 That HDL cholesterol is consistently associated with aortic wall thickness, independent from clinical markers of abdominal obesity, indicates that its protective effect against CVD is maintained across the continuum of body weight in African-Americans. This observation may be due to the well-established fact that African-Americans have higher HDL cholesterol levels than Caucasians,37, 38 but may also have important public health implications in other groups.
In this sample, SAT is inversely associated with distal aIMT in bi-variate analysis (Table 2), and VAT is inversely associated with distal aIMT in women in the multivariable analysis. Although the inverse association of SAT with distal aIMT may be due to confounding—as evidenced by the lack of significance in the adjusted model—the fact that both SAT and VAT appear to have and inverse relation with wall thickness of distal aorta deserves further discussion. Three explanations can be presented for this observation: First, it actually agrees with other reports suggesting a protective role of SAT on CVD risk.39–41 This could be due to SAT providing a natural storage for excess lipids and may play a role in reducing insulin resistance.40,41 However, it is not known whether a thinner distal aortic wall actually reflects a “protective” effect or has any clinical benefit. Second, the opposing effects of abdominal obesity on proximal and distal aorta could be due to the marked difference in wall structure of proximal and distal aorta, making them responding differently to alterations in CVD risk factors driven by abdominal obesity.42 Proximal aorta is abundant with elastin fibers while distal aorta is abundant with collagen fibers and has more vascular smooth muscle and other stromal cells.43 The close proximity of the ascending aorta to the heart makes it more exposed to the pressure wave originating from ventricular contraction and, consequently, more vulnerable to hemodynamic changes associated with obesity, such as increased blood pressure. This argument is supported by MAP being associated with proximal, but not distal, aorta in this sample. Third, the inverse relation of SAT and VAT with distal aIMT could be merely a chance finding due to the difficulty in measuring distal aorta because of its anatomical location behind abdominal fat. This may have caused a systematic negative shift in measuring distal aIMT as abdominal fat increased.
In this sample, smoking also shows an independent effect on aortic wall that tends to be more prominent on distal aorta. Finally, plasma triglycerides are inversely associated with proximal aortic wall thickness in the models of WC and WHtR (Table 3). We believe that this is a chance finding due to the opposite trajectories of the two variables over time, where triglycerides levels were decreasing while proximal aIMT was increasing. We examined this notion by comparing mean triglycerides at each visit (triglycerides = 106.4, 100.7, and 97.9 mg/dL at visits 1, 2, and 3, respectively; p<0.001).
The study has some limitations; the clinical importance of the observed associations is difficult to determine from the available data since information on relevant clinical outcomes (e.g., myocardial infarction or CHD) is not available. Selection bias may have occurred because only a subset of the cohort had complete data on aortic thickness and markers of abdominal obesity. A comparison between the study sample and the rest of the cohort revealed that the study sample is slightly younger, leaner, and had lower systolic blood pressure (mean age 52.6 vs. 56.3 years; WC 98.4 vs. 101.5 cm, and systolic blood pressure 123.2 vs. 128.2 mmHg). The two groups are similar, however, in gender distribution, income and education levels, and other hemodynamic and metabolic markers of CVD risk.
The study has several strengths; the large sample size, the long follow up data, the adjustment for many clinical, behavioral, and socio-economic CVD risk factors, and the assessment and comparison of multiple markers of abdominal obesity on three different segments of the aorta in the same sample.
CONCLUSION
In conclusion, the findings of this study indicate that abdominal obesity is associated with increased wall thickness in proximal, but not distal segments of the abdominal aorta in African-Americans. They also provide evidence of a protective effect of HDL cholesterol against increased aortic wall thickness in African-Americans, independent of measures of abdominal obesity. Finally, the findings suggest that WC may have a greater public health utility for identifying high-risk groups for CVD than VAT or SAT.
Supplementary Material
What is already known:
Abdominal obesity is associated with higher risk of cardiovascular disease
Wall thickness of the central arteries is associated with cardiovascular disease
African-Americans have higher prevalence of overweight and higher cardiovascular disease risk than any other ethnicity in the US
What does the study add:
Abdominal obesity is associated with wall thickness of both proximal and distal aorta in African-Americans
This suggests that aortic wall thickness plays a role in the observed association of abdominal obesity with cardiovascular risk in African-Americans
Waist circumference may have a better public health utility for identifying high-risk groups than other markers of abdominal obesity, owing to its association with wall thickness in all segments of aorta and its easy measurability.
ACKNOWLEDGEMENTS
The authors thank the participants and data collection staff of the Jackson Heart Study. This Manuscript is based on de-identified individual data provided by the Jackson Heart Study (JHS) according to a data-sharing agreement between the first author and the JHS team and is in compliance with the terms of that agreement. The Manuscript has been reviewed and approved by the JHS Publications and Presentation Committee.
Funding
The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD).
Footnotes
Disclosure
The authors declare no conflict of interest.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Anonymous. World Health Organization. Cardiovascular disease (CVDs). 2017;2017.
- 2.Anonymous. Centers of Disease Control and National Center of Health Statistics. Underlying Cause of Death 1999–2015. In Multiple Cause of Death Files, 1999–2015, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. 2017.
- 3.Bhuiyan AR, Srinivasan SR, Chen W, Paul TK and Berenson GS. Correlates of vascular structure and function measures in asymptomatic young adults: the Bogalusa Heart Study. Atherosclerosis. 2006;189:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz MW, Markus HS, Bots ML, Rosvall M and Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67. [DOI] [PubMed] [Google Scholar]
- 5.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL and Wolfson SK Jr, Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 6.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA and D’Agostino RB Sr, Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhassen L, Carville C, Pelle G, Monin JL, Teiger E, Duval-Moulin AM, Dupouy P, Dubois Rande JL and Gueret P. Evaluation of carotid artery and aortic intima-media thickness measurements for exclusion of significant coronary atherosclerosis in patients scheduled for heart valve surgery. J Am Coll Cardiol. 2002;39:1139–44. [DOI] [PubMed] [Google Scholar]
- 8.Jeltsch M, Klass O, Klein S, Feuerlein S, Aschoff AJ, Brambs HJ and Hoffmann MH. Aortic wall thickness assessed by multidetector computed tomography as a predictor of coronary atherosclerosis. Int J Cardiovasc Imaging. 2009;25:209–17. [DOI] [PubMed] [Google Scholar]
- 9.Frogoudaki A, Barbetseas J, Aggeli C, Panagiotakos D, Lambrou S, Pitsavos C and Stefanadis C. Thoracic aorta atherosclerosis burden index predicts coronary artery disease in patients undergoing transesophageal echocardiography. Atherosclerosis. 2008;197:232–6. [DOI] [PubMed] [Google Scholar]
- 10.Schachner T, Zimmer A, Nagele G, Hangler H, Laufer G and Bonatti J. The influence of ascending aortic atherosclerosis on the long-term survival after CABG. Eur J Cardiothorac Surg. 2005;28:558–62. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Berry JD, Ayers CR, Peshock RM, Khera A, de Lemos JA, Patel PC, Markham DW and Drazner MH. Left ventricular hypertrophy, aortic wall thickness, and lifetime predicted risk of cardiovascular disease:the Dallas Heart Study. JACC Cardiovascular imaging. 2010;3:605–13. [DOI] [PubMed] [Google Scholar]
- 12.Biancari F, Lahtinen J and Heikkinen J. Impact of ascending aortic wall thickness and atherosclerosis on the intermediate survival after coronary artery bypass surgery. European Journal of Cardio-Thoracic Surgery. 2012;41:e94–e99. [DOI] [PubMed] [Google Scholar]
- 13.Balkau B, Deanfield JE, Despres JP, Bassand JP, Fox KA, Smith SC Jr., Barter P, Tan CE, Van Gaal L, Wittchen HU, Massien C and Haffner SM. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116:1942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Rexrode KM, van Dam RM, Li TY and Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–67. [DOI] [PubMed] [Google Scholar]
- 15.Abraham TM, Pedley A, Massaro JM, Hoffmann U and Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JD, Borel AL, Nazare JA, Haffner SM, Balkau B, Ross R, Massien C, Almeras N and Despres JP. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. J Clin Endocrinol Metab. 2012;97:1517–25. [DOI] [PubMed] [Google Scholar]
- 17.Ryden M and Arner P. Cardiovascular risk score is linked to subcutaneous adipocyte size and lipid metabolism. J Intern Med. 2017;282:220–228. [DOI] [PubMed] [Google Scholar]
- 18.Ferdinand KC. Coronary artery disease in minority racial and ethnic groups in the United States. American Journal of Cardiology. 2006;97:12a–19a. [DOI] [PubMed] [Google Scholar]
- 19.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ and Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. [DOI] [PubMed] [Google Scholar]
- 20.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M and Hong Y. Heart disease and stroke statistics−−2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. [DOI] [PubMed] [Google Scholar]
- 21.Kwagyan J, Retta TM, Ketete M, Bettencourt CN, Maqbool AR, Xu S and Randall OS. Obesity and Cardiovascular Diseases in a High-Risk Population: Evidence-Based Approach to CHD Risk Reduction. Ethn Dis. 2015;25:208–13. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Coady S, Carr JJ, Hoffmann U, Taylor HA and Fox CS. Differential associations of abdominal visceral, subcutaneous adipose tissue with cardiometabolic risk factors between African and European Americans. Obesity (Silver Spring). 2014;22:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu CY, Chen D, Bluemke DA, Wu CO, Teixido-Tura G, Chugh A, Vasu S, Lima JA and Hundley WG. Evolution of aortic wall thickness and stiffness with atherosclerosis: long-term follow up from the multi-ethnic study of atherosclerosis. Hypertension. 2015;65:1015–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ and Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2011;31:2715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Musani SK, Bidulescu A, Carr JJ, Wilson JG, Taylor HA and Fox CS. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel VG, Gupta DK, Terry JG, Kabagambe EK, Wang TJ, Correa A, Griswold M, Taylor H and Carr JJ. Left Ventricular Function Across the Spectrum of Body Mass Index in African Americans: The Jackson Heart Study. JACC Heart Fail. 2017;5:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor HA Jr., Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C and Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study Ethn Dis. 2005;15:S6–4–17. [PubMed] [Google Scholar]
- 28.Koskinen J, Kahonen M, Viikari JS, Taittonen L, Laitinen T, Ronnemaa T, Lehtimaki T, Hutri-Kahonen N, Pietikainen M, Jokinen E, Helenius H, Mattsson N, Raitakari OT and Juonala M. Conventional cardiovascular risk factors and metabolic syndrome in predicting carotid intima-media thickness progression in young adults: the cardiovascular risk in young Finns study. Circulation. 2009;120:229–36. [DOI] [PubMed] [Google Scholar]
- 29.Kardassis D, Schonander M, Sjostrom L and Karason K. Carotid artery remodelling in relation to body fat distribution, inflammation and sustained weight loss in obesity. J Intern Med. 2014;275:534–43. [DOI] [PubMed] [Google Scholar]
- 30.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB Sr. and O’Donnell CJ Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 31.Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P and Investigators ftHA. Aortic Stiffness Is Associated With Visceral Adiposity in Older Adults Enrolled in the Study of Health, Aging, and Body Composition. Hypertension. 2001;38:429–433. [DOI] [PubMed] [Google Scholar]
- 32.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL Jr., Ravussin E, Ryan DH, Smith SR and Bouchard C. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinsier RL, Hunter GR, Gower BA, Schutz Y, Darnell BE and Zuckerman PA. Body fat distribution in white and black women: different patterns of intraabdominal and subcutaneous abdominal adipose tissue utilization with weight loss. Am J Clin Nutr. 2001;74:631–6. [DOI] [PubMed] [Google Scholar]
- 34.Bajaj HS, Brennan DM, Hoogwerf BJ, Doshi KB and Kashyap SR. Clinical utility of waist circumference in predicting all-cause mortality in a preventive cardiology clinic population: a PreCIS Database Study. Obesity (Silver Spring). 2009;17:1615–20. [DOI] [PubMed] [Google Scholar]
- 35.Lorbeer R, Schneider T, Quadrat A, Kühn J-P, Dörr M, Völzke H, Lieb W, Hegenscheid K and Mensel B. Cardiovascular Risk Factors and Thoracic Aortic Wall Thickness in a General Population. Journal of Vascular and Interventional Radiology. 2015;26:635–641. [DOI] [PubMed] [Google Scholar]
- 36.Alagona C, Soro A, Westerbacka J, Ylitalo K, Salonen JT, Salonen R, Yki-Jarvinen H and Taskinen MR. Low HDL cholesterol concentration is associated with increased intima-media thickness independent of arterial stiffness in healthy subjects from families with low HDL cholesterol. Eur J Clin Invest. 2003;33:457–63. [DOI] [PubMed] [Google Scholar]
- 37.Glueck CJ, Gartside P, Laskarzewski PM, Khoury P and Tyroler HA. High-density lipoprotein cholesterol in blacks and whites: potential ramifications for coronary heart disease. Am Heart J. 1984;108:815–26. [DOI] [PubMed] [Google Scholar]
- 38.Sprafka JM, Norsted SW, Folsom AR, Burke GL and Luepker RV. Life-style factors do not explain racial differences in high-density lipoprotein cholesterol: the Minnesota Heart Survey. Epidemiology. 1992;3:156–63. [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin T, Lamendola C, Liu A and Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ and Fox CS. Abdominal Subcutaneous Adipose Tissue: A Protective Fat Depot? Diabetes Care. 2009;32:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ and DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–91. [DOI] [PubMed] [Google Scholar]
- 42.Safar ME, Levy BI and Struijker-Boudier H. Current Perspectives on Arterial Stiffness and Pulse Pressure in Hypertension and Cardiovascular Diseases. Circulation. 2003;107:2864–2869. [DOI] [PubMed] [Google Scholar]
- 43.Nichols WW ORM, Vlachopoulos C. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. Sixth ed London UK: CRC Press; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.