Figure 7. Schematic illustrating the ‘latching switch’ mechanism of Rho-catalyzed nucleotide exchange in GT.
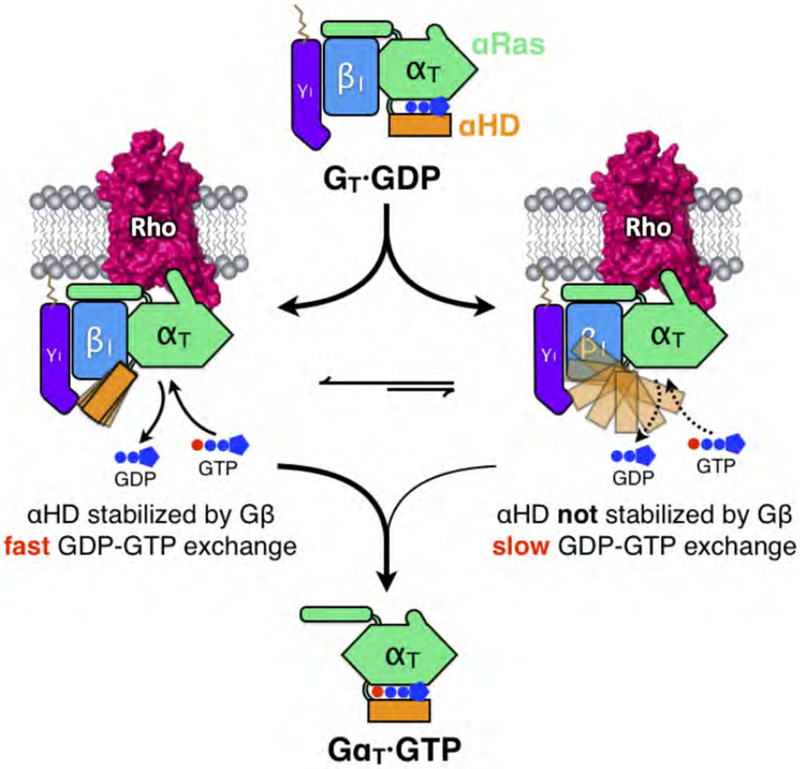
Coupling with Rho disengages αHD from the Ras domain and increases the flexibility of αHD. In a significant population of the Rho-GT complex particles, Gβ latches onto αHD and stabilizes it at an open conformation through a series of electrostatic interactions. These interactions prolong the opening of the nucleotide-binding pocket providing a clear exit route for GDP, while also positioning the αHD in close proximity to the Ras-like domain to facilitate quick closure of the clam-shell-like structure upon GTP loading. The Gβ-αHD interactions allow for fast GDP-GTP exchange and when these interactions are disrupted in the αHD mutants, Rho-catalyzed nucleotide exchange activity is markedly reduced.
