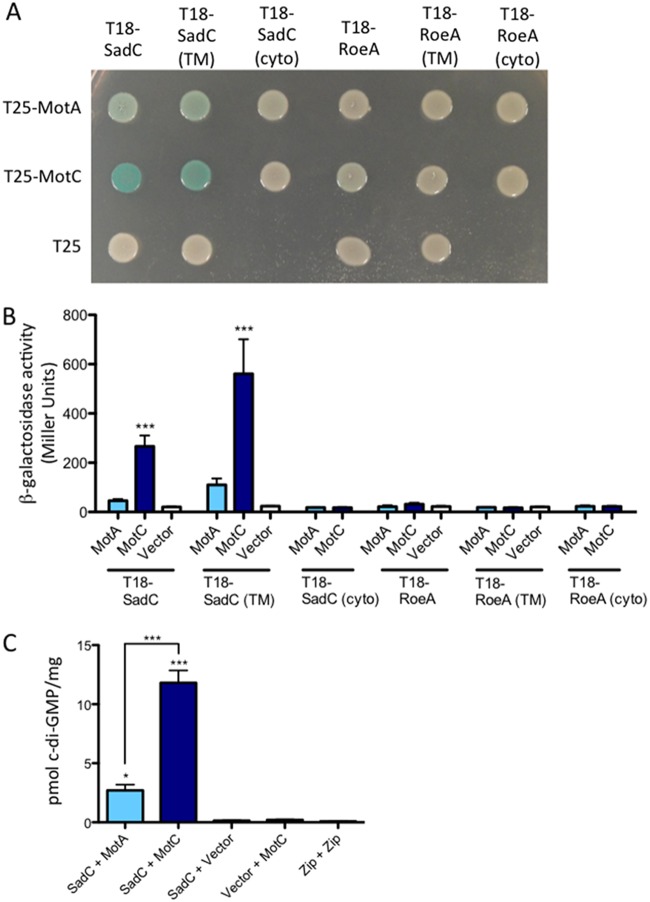
FIG 3.
Detection of a physical interaction between MotC and SadC by bacterial two-hybrid analysis. (A) The sadC and roeA genes (and truncated versions) were cloned into the vector pUT18C. “TM” indicates the predicted transmembrane domain, and “cyto” indicates the predicted cytoplasmic region. The motA and motC genes were cloned into the vector pKT25. Plasmids were cotransformed into E. coli BTH101 cells. The transformants were spotted (2 μl) onto LB agar containing Cb, Kan, IPTG, and X-Gal. Plates were incubated at 30°C for 40 h. Cleavage of X-Gal (blue) indicates a positive protein-protein interaction. (B) Bacterial two-hybrid interactions were quantified by measuring β-galactosidase activity of transformants grown at 30°C overnight in LB broth supplemented with Cb and Kan. “Vector” indicates an empty pKT25 vector. The data represent results from 2 experiments, each with 2 to 5 replicates. Values are means ± SEM. Significance was determined by analysis of variance and Dunnett’s posttest comparison for differences from the negative control (T18-SadC’s Vector bar). ***, P < 0.001. (C) Quantification of cellular c-di-GMP levels by LC-MS from B2H assays. The x axis displays the two fusion proteins (listed, in order, as pUT18C and pKT25) cotransformed into BTH101 cells. After being cotransformed with 2 fusion plasmids, cells were spotted onto LB agar containing Cb, Kan, and IPTG (without X-Gal). Plates were incubated at 30°C for 40 h, and then cells were scraped off plates and nucleotides were extracted. Data are expressed as picomoles of c-di-GMP per milligram (dry weight) of cells from which nucleotides were extracted. The data represent results from 2 experiments, each performed in triplicate. Values are means ± SEM. Significance was determined by analysis of variance and Tukey’s post hoc test comparison for differences from the negative control (T18-SadC + Vector) and differences between the indicated samples. Zip + Zip, positive control for the B2H assay. *, P < 0.05; ***, P < 0.001.