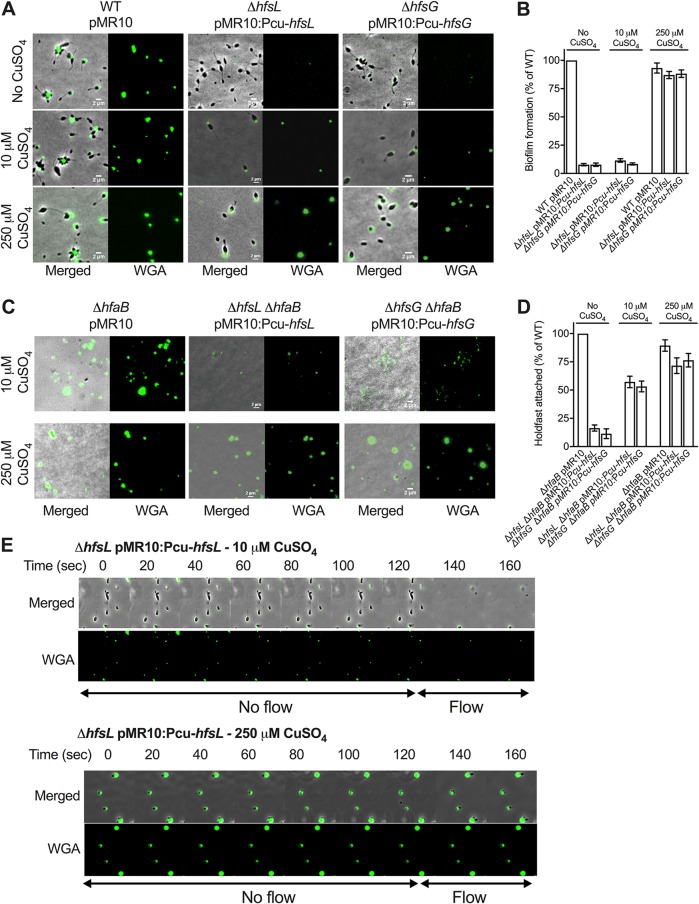
FIG 3.
Effect of modulating hfsL and hfsG expression on H. baltica holdfast properties. (A) Representative images showing merged phase and fluorescence channels of H. baltica WT and ΔhfsL and ΔhfsG mutants complemented with copper-inducible promoter constructs and grown in marine broth with 0 μM,10 μM, and 250 μM CuSO4. The holdfasts were labeled with WGA-AF488. (B) Biofilm quantification after 12 h using crystal violet assay of ΔhfsL and ΔhfsG mutants and complementations under copper-inducible promoters in marine broth supplemented with 0 μM, 10 μM, and 250 μM CuSO4. The data are expressed as averages from 6 independent replicates, and the error bars represent the standard errors. (C) Images of WGA-AF488-labeled H. baltica ΔhfaB, H. baltica ΔhfaB ΔhfsL pMR10:PcuhfsL, and H. baltica ΔhfaB ΔhfsG pMR10:PcuhfsG shed holdfasts bound to glass slides. Cells were grown in marine broth with 0 μM, 10 μM, and 250 μM CuSO4 induction for 4 h. (D) Percentages of holdfasts bound to glass slides per field of view at different CuSO4 induction levels shown in panel C. The data are expressed as averages from 5 independent replicates, and the error bars represent the standard errors. (E) Time-lapse montage of H. baltica ΔhfsL pMR10:PcuhfsL induced with 10 μM (top) and 250 μM (bottom) CuSO4 in a microfluidic device, initially with no flow and then with a flow of 1.4 μl/min, generating a drag force of 4 nN, introduced into the microfluidic device. The arrows indicate the times when no flow (first 120 s) and flow (later times) were applied to the device.