Abstract
Objectives
Stress-induced peptic ulcer disease (SPUD) refers to erosions in the mucosa of the upper gastrointestinal tract that are caused by stress. Some antidepressants are reported to have antioxidant and antiulcer effects. However, histopathological and biochemical evaluation of the anti-ulcer activity of a comparable antidepressant, fluvoxamine, has not been adequately investigated. This study aims to determine the anti-ulcer efficacy of fluvoxamine in reducing stress-induced histopathological and biochemical changes in the gastric mucosa.
Methods
Thirty adult male albino rats were divided into three groups of 10 rats each: the control groups, the SPUD group, and the fluvoxamine-pre-treated group, which received fluvoxamine for eight days before stress exposure. The cold-restraint stress method was used to induce stomach ulcers in the SPUD and fluvoxamine groups. Afterward, the stomachs of rats were removed, opened, and ulcer indices were calculated. Light microscopy was performed following haematoxylin and eosin staining, periodic acid Schiff's, Masson's trichrome staining, and proliferating cell nuclear antigen immunostaining. Gastric tissue levels of oxidative stress markers were measured and compared among groups.
Results
The stomachs of the fluvoxamine-treated rats showed a significantly lower number of ulcers with minimal mucosal injury compared with those of rats from the SPUD group. The oxidative stress marker levels and SPUD ulcer indices were significantly different among groups.
Conclusion
Fluvoxamine pre-treatment exerted a gastroprotective effect against ulcer development and promoted healing of the developed lesions.
Keywords: Fluvoxamine, Gastric ulcer, Peptic ulcer, Stress, Stress-induced peptic ulcer
الملخص
أهداف البحث
يشير مصطلح "القرحة الهضمية الناجمة عن الإجهاد" إلى وجود جروح في الغشاء المخاطي للجهاز الهضمي العلوي نتجت عن الإجهاد. وقد تم تسجيل آثار مضادة للأكسدة ومضادة للتقرّح لبعض مضادات الاكتئاب. إلا أن التقييم النسيجي والتقييم الكيميائي الحيوي للنشاط المضاد للتقرّح لمضاد اكتئاب مشابه، هو " فلوفوكسامين" لم يتم التحري عنهما بصورة كافية. تهدف الدراسة إلى تحديد الفاعلية المضادة للتقرّح لـ "فلوفوكسامين" في إحداث التغيرات النسيجية والكيميائية الحيوية الناجمة عن الإجهاد في الغشاء المخاطي في المعدة.
طرق البحث
تم تقسيم ثلاثين من ذكور الجرذان البيضاء البالغة إلى ثلاث مجموعات من ١٠ جرذان؛ المجموعة الضابطة ومجموعة "القرحة الهضمية الناجمة عن الإجهاد" والمجموعة سابقة المعالجة بـ "فلوفوكسامين" التي تلقت "فلوفوكسامين" لمدة ثمانية أيام قبل التعرض للإجهاد. تم تعريض مجموعة "القرحة الهضمية الناجمة عن الإجهاد" ومجموعة "فلوفوكسامين" إلى طريقة منع الحركة الباردة لاستحداث قُرَح في المعدة. بعد ذلك تم استئصال معداتهم وفتحها واحتساب مؤشرات التقرح. تم فحص العينات بعد صبغها بصبغة "هيموتوكسيلين وإيوسين" وصبغة "بي أي اس" وصبغة "ماسون ثلاثية الألوان" وصبغة "بي سي إن أي" المناعية بالمجهر الضؤئي. وتم قياس مستويات علامات الإجهاد التأكسدي في النسيج المعدي ومقارنته بين المجموعات.
النتائج
أظهرت مَعِدات المجموعة سابقة المعالجة بـ "فلوفوكسامين" عدد قُرح أقل بشكل ملحوظ مع حد أدنى من إصابة الغشاء المخاطي مقارنة بالمجموعة المصابة. وأظهر التحسن في مستويات علامات الإجهاد التأكسدي وفي علامات مؤشر قرحة مجموعة "القرحة الهضمية الناجمة عن الإجهاد" فرقا كبيرا بين المجموعات.
الاستنتاجات
فلوفوكسامين كان له أثر معزز للمعدة ضد نشوء القُرح وساعد على شفاء القُرح الموجودة.
الكلمات المفتاحية: فلوفوكسامين, الإجهاد, القرحة الهضمية, قرحة المعدة, القرحة الهضمية الناجمة عن الإجهاد
Introduction
Peptic ulcer disease (PUD) is a common disease worldwide.1 PUD occurs as a defect in the mucosa of the stomach or duodenum that exceeds the muscularis mucosa.2 PUD follows gastric mucosal injuries as a result of imbalance between the defensive and the aggressive factors affecting the mucosa.3, 4 Many factors contribute to the development of PUD, of which environmental factors such as psychosocial conditions and stress are the most outstanding.5
Stress is an acute hazard/risk to homeostasis that excites an allostatic or adaptive response. Stress affects the function of the gastrointestinal tract either in short or long-term impacts.5 Studies revealed that stress contributes to the formation of PUD and is frequently used to produce PUD in experimental animal models.6
Stress-induced peptic ulcer disease (SPUD) or stress-related gastric mucosal lesions occur as a typical stress-induced organ injury.7 SPUD incidence is increasing worldwide and is considered a significant cause of pain and distress, with an accompanying impairment of quality of life.8 The onset and modulation of SPUD may be caused by various types of stress,9 of which several types of major physiologic harms, including trauma, CNS injury, burn injury, major surgical procedures and critical illnesses are the most common.10 The pathogenesis of SPUD is not clearly discussed in previous research, but it differs from ordinary PUD in symptoms and risk factors.7 It has been suggested that in SPUD there is insufficient blood microcirculation, which results in accumulation of reactive oxygen species (ROS) with accompanying lipid peroxidation and subsequent loss of normal cellular functions.11
Being a serious gastrointestinal disorder, PUD demands a well-targeted therapeutic approach.12 Numerous drugs are available for the treatment of PUD, involving anti-acids, H2 receptor antagonists, and proton pump inhibitors13; however, clinical evaluation has revealed major side effects, drug interactions, and incidences of relapse from these drugs.14
Tricyclic antidepressants have been reported to be used for their antioxidant effects.15 They were the first antidepressants used for the treatment of PUD.16 Although some selective serotonin reuptake inhibitor (SSRI) drugs have gastric side effects that might progress to gastrointestinal bleeding if used in combination with indomethacin,17, 18 over time, other antidepressants have been shown to possess variable degrees of anti-ulcer action.19, 20, 21 Alternatively, novel anxiolytics have gastroprotective effects in experimental animals.22 Some drugs have shown other, more beneficial GIT effects, such as increasing gastric contractility23 and decreasing stomach and intestinal distension.24
Fluvoxamine is an SSRI broadly prescribed for depression25 and is used for treating obsessive-compulsive disorder.26 This drug inhibits CYP1A2,25 an enzyme known to be involved in ROS generation.27 Unlike most SSRIs, which enhance upper GIT bleeding, fluvoxamine is postulated to be beneficial in the management of PUD.
The combined antioxidant and antidepressant effects of fluvoxamine favours its use in treatment of SPUD. However, the histopathological and biochemical changes associated with its anti-ulcer activity are not fully elucidated. Thus, the aims of this study is to examine the histopathological and biochemical changes in the gastric mucosa induced by stress, and to investigate the effects of fluvoxamine on stress-induced ulcers.
Materials and Methods
Thirty adult male eleven-week-old albino rats (130–150 g average body weight), obtained from the Mansoura Animal House were used in this study. Animals were housed in the Mansoura Faculty of Medicine Animal House under standard laboratory conditions. Commercial standard pellet diet was used for feeding, with free access to food and water. The animals were acclimatized to standard laboratory conditions (according to Mansoura University IRB protocols); the temperature was 20 ± 1 °C, with a 12:12-h light–dark cycle for 10 days before the experiment. To prevent coprophagy, a grid floor was placed in each cage. The animals were randomly assigned to three groups of 10 animals each: Group I (the control group), Group II (the SPUD group), and Group III (the fluvoxamine-treated group). Groups I and II received sterile water, while Group III received fluvoxamine solution by an orogastric tube for 8 days before stress induction.
The fluvoxamine solution was prepared by dissolving 50 mg film-coated fluvoxamine tablets (Solvay, Cairo, Egypt) in sterile water. The solution was prepared just prior to dosing at a concentration of 50 mg/kg,21 and administered daily to 12-h fasted rats by an orogastric tube as a pre-treatment (for 1 day) and repeated for 7 consecutive days.
Induction of SPUD in 12 h-fasted rats of Groups II and III using the cold immobilization restraint method as previously described.28 Rats were tied to a wooden plank and immersed individually in cold water (6 ± 0.6 °C) for 6 h. The same procedure was repeated daily for 7 days.7 At the assigned time, the animals were sacrificed under diethyl ether anaesthesia, the abdomens were opened at the midline and the stomachs were gently removed, washed with saline, opened at the greater curvature, and photographed with a digital camera (Canon 650D).
The total ulcer surface area was measured from the photographs after considering the drawing scale. Ulcer severity was scored by the sum of the total ulcer surface area in the glandular portion of the stomach by a person blind to the experimental conditions. The gastric lesions were scored between 1 and 6 according to their severity. The calculation of the ulcer score was performed according to Palle et al.29 The index was calculated by multiplying the average number of ulcers per stomach by the ulcer severity score and the percentage of animals with ulcers.
The stomachs were divided into two equal halves (at the lesser curvature): one for microscopic examination and the other for biochemical assessment. For light microscopic examination, specimens were prefixed in 10% neutral buffered formalin and processed for staining with haematoxylin and eosin (H&E), Masson's trichrome, and Periodic Acid Schiff's (PAS) stains.30 Proliferating cell nuclear antigen (PCNA) immunohistochemical staining was performed (a three-step immunoperoxidase staining technique) using mouse monoclonal anti-PCNA/cyclin antibody (Dako, clone PC-10) purchased from Heliopolis, Cairo, Egypt, with haematoxylin counterstaining.31
For biochemical assessments, the stomach halves were homogenized with 0.1 M phosphate saline buffer, filtered, centrifuged, and stored according to the standards.29 The supernatants were used for determination of oxidative enzymes (lipid peroxidation (LPO), catalase (CAT), superoxide dismutase (SOD)), and reduced glutathione (GSH) using commercially available kits (Sigma-Aldrich, Riyadh KSA) by the usual techniques.32, 33, 34
Each experimental value was expressed as the mean ± SD. One-way analysis of variance (ANOVA) was performed to compare different groups. A mean difference was considered significant at P < 0.05. The Bonferroni multiple range test was used as a post-hoc test.
Results
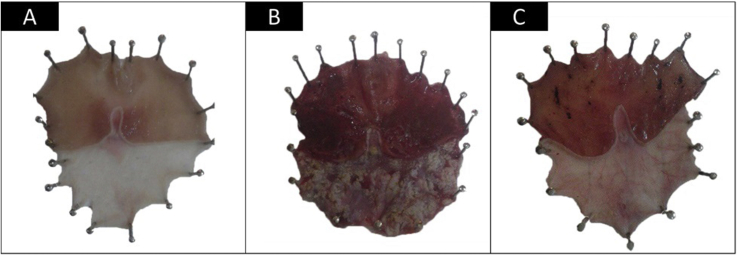
Photographs of the morphological examinations of the stomachs are shown in Figure 1. Group I stomachs showed apparently normal mucosa without signs of inflammation, haemorrhage or hyperaemia (Figure 1A). On the other hand, Group II showed marked severe injuries in the gastric mucosa in the form of severe haemorrhagic patches in both the fundic and corporeal regions of the stomachs and less marked patches in the antra. The ulcers appeared as dark reddish patches of variable forms and sizes with generalized hyperaemia in the gastric mucosa (Figure 1B). Group III stomachs showed fewer ulcers with less marked hyperaemia and moderate mucosal injuries. Ulcers appeared smaller, widely spaced, and discontinuous. Lesions in the gastric mucosa were limited to the corporeal region, while the fundus appeared normal (Figure 1C). None of the animals showed perforation lesions.
Figure 1.
Photographs of gross ross appearance of the gastric mucosa. (A) Group I; (B) Group II; (C) Group III.
Histological examination
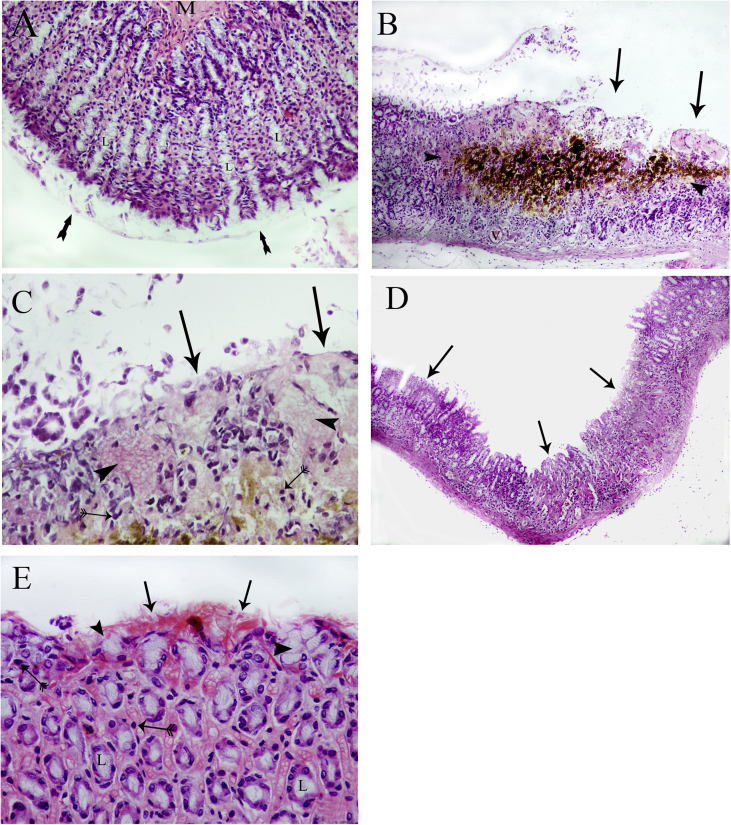
H&E staining of Group I samples revealed normal gastric mucosal layers resting on the submucosa. The mucosa is thrown into folds formed of columnar ciliated epithelium. The apparent normal lamina propria, with its contained gastric glands, and the muscularis mucosa was observed (Figure 2A). Group II samples showed areas of sloughing of the gastric mucosa. The gastric glands showed vacuolar degeneration and areas of necrosis, with dilated gastric pits with inflammatory cell infiltration into the submucosa. Areas of haemorrhage were evident in the submucosa, with congestion of the nearby blood vessels (Figure 2B and C). Group III samples showed nearly normal gastric mucosa, with signs of regeneration in the gastric glands, but inflammatory cell infiltration was still evident (Figure 2D and E).
Figure 2.
A) Photomicrograph of a section of the fundic region of a representative control group rat. The mucosa has an intact surface epithelial covering (arrows), gastric glands stuffed in the lamina propria (L), and the muscularis mucosa (M) appears normal (H&E X100). B) Group II rat fundus showing patches of sloughing of the gastric mucosal epithelium (arrows) with signs of lining cell desquamation and degeneration of the gastric glands, which appear vacuolated (Arrow heads). The submucosa has a distinct area of haemorrhage with congestion of the nearby blood vessels (V) (H&E X100). C) Higher magnification of the submucosa showing patches of sloughing of the gastric mucosal epithelium (arrows) with signs of lining cell desquamation and degeneration of the gastric glands, which appear vacuolated (Arrow heads), and infiltration of multiple mononuclear inflammatory cells (double-tailed arrows) (H&E X200). D) Group III rat fundic sections showing a more-or-less normal gastric mucosa with a small area of the surface epithelial atrophy still apparent (arrows) (H&E X100). E) Higher magnification showing a more-or-less normal gastric mucosa, with a small area of surface epithelial atrophy still apparent (arrows) and regenerating gastric glands (L) with fewer marked signs of cytoplasmic vacuolation (arrow heads). Mononuclear cell infiltration is still evident in the submucosa (double-tailed arrows) (H&E X400).
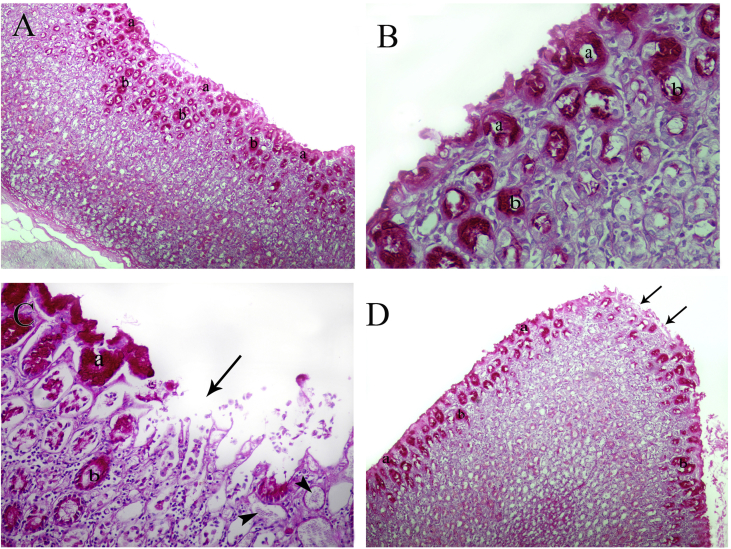
Group I samples showed strong PAS staining of the mucosal cells of both the surface and the neck of the gastric glands (Figure 3A and B). Group II samples showed areas of mucosal shedding, and weaker PAS reactivity (Figure 3C). Group III samples had a continuous, nearly normal mucosal covering, with PAS-reactive mucosal cells apart from small areas where reactivity was absent (Figure 3D).
Figure 3.
A) Control rat fundus showing strongly positive PAS reactivity in surface mucosal cells (a) and the neck regions of the gastric glands (b) (PAS X100). B) Higher magnification showing strong positive PAS reactivity in surface mucosal cells (a) and the neck regions of the gastric glands (b) (PAS X400). C) Group II rat fundus showing an area of interruption of the mucosa (arrow) with mucoid degeneration in some glands (arrow heads) and weak PAS reactivity in surface mucosal cells (a) and the neck regions of the gastric glands (b) (PAS X400). D) Group III rat fundus, showing positive PAS reactivity in surface mucosal cells (a) and the neck regions of the gastric glands (b) apart from small areas of non-reactivity (arrows) (PAS X100).
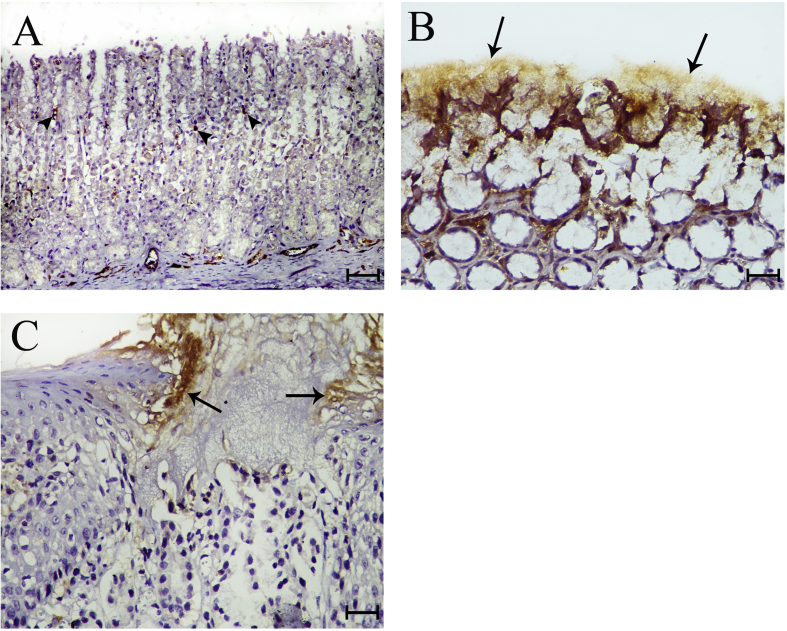
Immunohistochemical staining of the control group revealed weak expression of PCNA in the neck cells of the gastric glands (Figure 4A). The reactivity was moderate in the glandular epithelium of Group II samples (Figure 4B). In Group III samples, the reactivity was mild and limited to the gland neck cells (Figure 4C).
Figure 4.
A) Control rat fundus showing weak PCNA expression in the neck cells of the gastric mucosa (arrow heads) (PCNA X100). B) Group II rat fundus showing marked expression of PCNA in the epithelia of the mucus glands (arrows) (PCNA X400). C) Group III rat fundus showing mild PCNA expression in the epithelia of the mucus glands at the neck region (arrows) (PCNA X400).
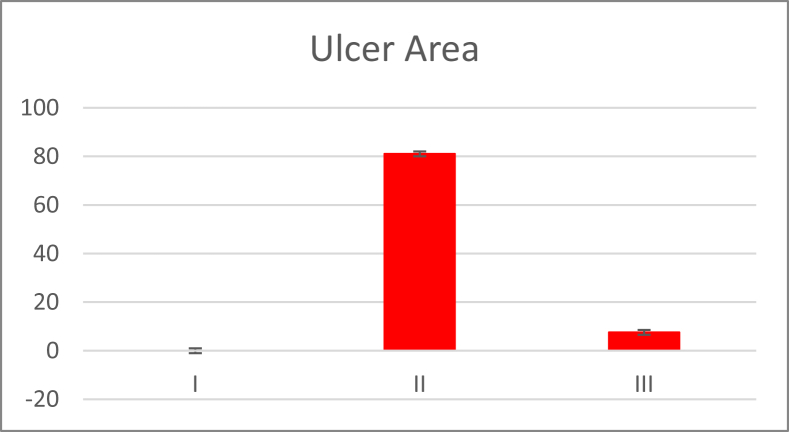
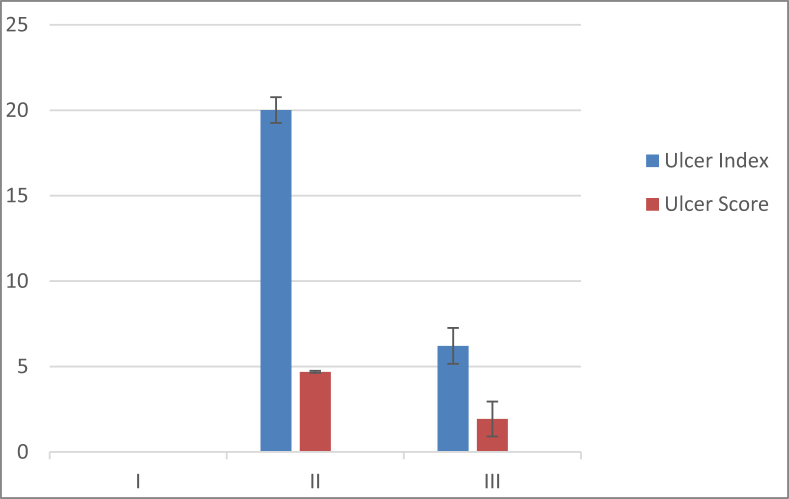
The effect of fluvoxamine pretreatment on SPUD ulcer index parameters (n = 10, X ± SEM) significantly increased the ulcer index, score, and area in Group III samples (6.21 ± 1.02, 1.93 ± 0.09 and 7.53 ± 0.63) compared with those in Group II samples (20.01 ± 1.08, 4.69 ± 0.06 and 81.02 ± 9.21) (P < 0.05) (Table 1 and Diagram 1, Diagram 2).
Table 1.
Morphometric measurements of the ulcer areas, indices, and score.
| Group | Ulcer area (In mm2) | Ulcer index | Ulcer score |
|---|---|---|---|
| I | 0 | 0 | 0 |
| II | 81.02 ± 9.21 | 20.01 ± 1.08 | 4.69 ± 0.06 |
| III | 7.53 ± 0.63* | 6.21 ± 1.02* | 1.93 ± 0.09* |
I = Control group, II = Stress-induced peptic ulcer disease group, III = Fluvoxamine-treated group.
Data are represented as the mean ± standard deviation.
*P < 0.05 versus Group II.
Diagram 1.
Ulcer area of the three groups in mm2. I = Control group, II = Stress-induced peptic ulcer disease (SPUD) group, III = Fluvoxamine-treated group.
Diagram 2.
Ulcer index and score of the three groups. I = Control group, II = Stress-induced ulcer (SPUD) group, III = Fluvoxamine treated group.
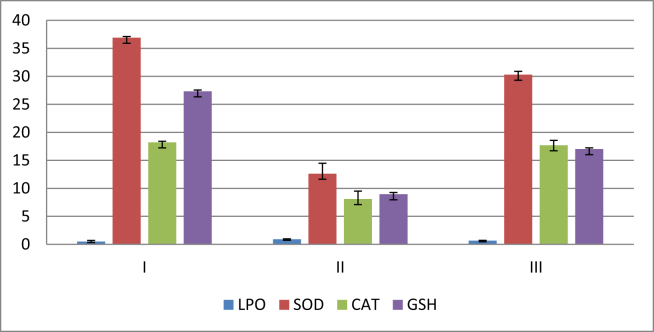
Group II rats showed significantly higher levels of LPO (0.91 ± 0.07) and lower levels of SOD and CAT (12.62 ± 1.86 and 8.09 ± 1.41, respectively) than did rats from Groups I and III (P < 0.05). Conversely, fluvoxamine pretreatment significantly decreased LPO (0.65 ± 0.05) and increased the SOD and CAT levels (30.3 ± 0.59 and 17.71 ± 0.85, respectively). Their levels were closer to control values, as shown in Table 2. The GSH level of Group II was significantly lower than that of Group I (8.95 ± 0.31). Group III showed a low but significant level (17.01 ± 0.21) (P < 0.05) Table 2 and Diagram 3).
Table 2.
Effect of fluvoxamine on stress-induced peptic ulcer disease biochemical parameters: lipid peroxidation (LPO), catalase (CAT), and superoxide dismutase (SOD).
| Group | LPO (nmol MDA/mg protein) | SOD (u/mg protein) | CAT (μmol H2O2/min/g protein) | GSH (μ mol/g tissue) |
|---|---|---|---|---|
| I | 0.50 ± 0.019 | 36.9 ± 0.22 | 18.22 ± 0.18 | 27.33 ± 0.22 |
| II | 0.91 ± 0.07* | 12.62 ± 1.86* | 8.09 ± 1.41* | 8.95 ± 0.31* |
| III | 0.65 ± 0.05** | 30.3 ± 0.59** | 17.71 ± 0.85** | 17.01 ± 0.21** |
I = Control group, II = Affected group, III = Treated group.
Data are represented as the mean ± standard deviation.
*P < 0.05 versus Group I. **P < 0.05 versus Group II.
Diagram 3.
Effect of fluvoxamine on stress-induced peptic ulcer disease biochemical parameters: lipid peroxidation (LPO), catalase (CAT), and superoxide dismutase (SOD). Data are represented as the mean ± standard deviation. I = Control group, II = Stress-induced peptic ulcer disease (SPUD) group, III = Fluvoxamine treated group.
Discussion
Stress is known as a significant participant in ulcer pathogenesis.3 Older studies attributed SPUD to excessive histamine release, which enhances acid secretion and reduces mucus production.35 Other studies reported an increase in gastric motility, vagal overactivity,36 mast cell degranulation,37 and decreased prostaglandins release.38
SPUD pathogenesis involves insufficient blood microcirculation with subsequent tissue hypoxemia and oxidative stress, with accumulation of ROS, which are known inducers of apoptosis.11 ROS disrupt the chemical structures of cell proteins, including DNA and lipids, and induce cell death in various ways. ROS also act as signalling molecules that control tissue responses to cell injury and inflammation.8
The body defends against ROS by both enzymatic and non-enzymatic defence mechanisms. The non-enzymatic defence mechanisms are evaluated by histopathological examination, and enzymatic defence mechanisms by biochemical examination.
Our results showed that exposure of the animals to cold stress induced shedding of the gastric protective layer with vacuolar degeneration of the gastric glands. Similar results were reported by Guo et al.39 A clear demonstration of sloughing of the mucosal lining and submucosal haemorrhage was a characteristic of SPUD. This finding is in accordance with the study of Ahmad et al.7 They explained these tissue changes by the altered gastric mucosal microcirculation,40, 41 with subsequent disturbances of gastric secretions42, 43 and motility.44 ROS and leukotrienes were postulated to mediate the cascade of stress-induced mucosal injury.45 The resulting superoxides in the tissues lead to tissue damage by promoting lipid peroxidation.46, 47 Previous studies reported neutrophil infiltration into the gastric mucosal tissues. This was explained by increased activities of myeloperoxidase and NO synthase.48, 49
Conversely, fluvoxamine treated animals exhibited nearly normal gastric mucosa, with areas of epithelial atrophy and signs of gland regeneration. Persistent inflammatory cell infiltration detected in the submucosa can be explained by the fact that the healing processes of ulcers remains continuous and requires considerable time to be fully resolved.
Some researchers have attributed the anti-ulcerogenic activity of antidepressants by their ability to block leukotriene receptors or reduce histamine secretion from mast cells.45, 50 However, in our study, pretreatment with fluvoxamine protected the mucosa from excessive sloughing and haemorrhagic patches. A reduction in the degree of damage of the stomach wall glandular tissue was noted. These findings were confirmed by our statistical analysis of the ulcerated areas, indices, and scores. It was clear that intact mucosa provides protection for the underlying tissues, including inner glandular and muscular layers.7
Mucopolysaccharides in the gastric mucosa are visualised by PAS staining.51 The weak PAS reactivity revealed that the glycoprotein content of the gastric mucosa was reduced in Group II. Conversely, an upsurge in the glycoprotein was evident by the augmented level of PAS reactivity in Group III.
Ulcerative lesions in the stomach are always associated with weak PAS reactivity. Gastroprotective agent treatments are usually accompanied by increases in reactivity.52 Other researchers have reported increased PAS reactivity with increased size of mucous glands.53
PCNA is an important tissue proliferation marker. The immunohistochemical outcomes of Group II revealed down-regulation of PCNA compared with that in Group I or Group III. Polo et al.53 found similar results comparing acetic acid-induced PUD with cimetidine and essential oil-treated groups.
PCNA downregulation in Group III indicates that fluvoxamine treatment promoted healing of gastric ulcers. The increased PCNA reactivity accompanied increases in cellular proliferation, which is a sign of ulcer re-epithelialisation.53 The increased mucosal barrier, indicated by enhanced PAS reactivity, and the increased cellular proliferation, indicated by enhanced PCNA staining, positively correlates with ulcer healing. Fluvoxamine not only maintained the gastric mucosa, but also promoted regeneration of the ulcerated regions. This effect is mostly produced by the cytoprotective effects of this drug in maintaining the integrity of the gastric mucosal microcirculation.
Biochemical identification of decreased SOD and CAT levels was reported to the formation of ROS.29 These enzymes are produced by the body as an enzymatic defence to facilitate the breakdown of free radicals.54 Our results showed that SPUD is accompanied by a significant decrease in the levels of both SOD and CAT. Conversely, fluvoxamine reversed these changes. The significant improvements in SOD and CAT levels strongly suggest that fluvoxamine acts against ROS.
As a marker of lipid peroxidation, LPO showed a significantly decreased level in the fluvoxamine-treated group. The inhibition of lipid peroxidation in the gastric mucosa is always associated with tissue protection. LPO levels decrease in accordance with the increased free radical scavenging enzymes (SOD and CAT).55
Gastric GSH is one of the most abundant antioxidant enzymes.56 A strong relationship between GSH levels and the levels of ulcer severity has been reported.57 GSH acts by trapping ROS. The decrease in GSH level is a sign of increased tissue oxidative stress.58 In our study fluvoxamine significantly increased GSH levels, although GSH remained lower than levels measured in the controls. A similar finding is reported as supporting evidence of efficacy for most drugs and natural products with antioxidant gastroprotective effects.7, 59 Other studies showed that fluvoxamine inhibited the indomethacin-induced ulcers in rats21 by decreasing levels of oxidant parameters in stomach tissues, particularly gastric GSH.
The exact mechanism by which fluvoxamine suppresses oxidative stress is still unclear. However, fluvoxamine, being an SSRI drug, inhibits the enzyme CYP1A2, which is an inhibitor of ROS.25, 27 The neuroprotective effect of fluvoxamine was attributed to its potent antioxidant and anti-inflammatory effects.60 This action was explained by its ability to inhibit NADPH oxidase and nitric oxide synthase.61 Recent studies revealed that it has the ability to modulate genes related to redox pathways, which in turn stimulates antioxidant elements.62 Other researchers demonstrated that fluvoxamine interacts with the mitochondrial lipid bilayer and affects electron transport, thereby inhibiting oxidative phosphorylation.63 It was also reported that fluvoxamine induces its antioxidant effect in nerves by increasing serotonin levels.64
The expected future increase in the coexistence of stress and SPUD adds to the importance of the use of antidepressants with SPUD healing properties.21, 65 Other studies have shown that antidepressants65, 66 and anxiolytics67, 68 can significantly reduce stress-induced ulcer formation, sometimes with better results than the traditionally used anti-ulcer drugs such as H2 receptor antagonists and antacids.67 Our findings lend support to these studies. The combined findings of both non-enzymatic and enzymatic defence mechanisms demonstrate the gastroprotective activity of fluvoxamine through an antioxidant pathway.
Conclusion
Fluvoxamine exerts gastroprotective effects via reduction of mucosal atrophy, promotion of gland regeneration, and potentiation of mucous secretion through inhibition of gastric tissue oxidative stress. Our results show that fluvoxamine exerts both an indirect effect on ulcer development through inhibition of stress and direct effects on previously developed ulcers.
Recommendation
Fluvoxamine can be used as a pretreatment to suppress the development of SPUD.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All animal handling procedures were in accordance to Mansoura University IRB protocols.
Authors' contributions
WME conceived and designed the study, conducted research, provided research materials, and collected and organized data. AMA, BTA, GTR and RMT shared analyses and interpretations of data. All authors wrote initial and final drafts of the article and provided logistical support. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Leontiadis G.I., Sreedharan A., Dorward S., Barton P., Delaney B., Howden C.W. Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol Assess. 2007 Dec;11(51) doi: 10.3310/hta11510. iii-164. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan K., Salinas R.C. Peptic ulcer disease. Am Fam Physician. 2007 Oct 1;76(7):1005–1012. [PubMed] [Google Scholar]
- 3.Rosenstock S., Jorgensen T., Bonnevie O., Andersen L. Risk factors for peptic ulcer disease: a population based prospective cohort study comprising 2416 Danish adults. Gut. 2003 Feb;52(2):186–193. doi: 10.1136/gut.52.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y.C., Cheng C.W., Lee H.J., Chu H.C. Apple polyphenol suppresses indomethacin-induced gastric damage in experimental animals by lowering oxidative stress status and modulating the MAPK signaling pathway. J Med Food. 2017 Nov;20(11):1113–1120. doi: 10.1089/jmf.2017.3951. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia V., Tandon R.K. Stress and the gastrointestinal tract. J Gastroenterol Hepatol. 2005 Mar;20(3):332–339. doi: 10.1111/j.1440-1746.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- 6.Brzozowski T., Konturek P.C., Chlopicki S., Sliwowski Z., Pawlik M., Ptak-Belowska A. Therapeutic potential of 1-methylnicotinamide against acute gastric lesions induced by stress: role of endogenous prostacyclin and sensory nerves. J Pharmacol Exp Ther. 2008 Jul;326(1):105–116. doi: 10.1124/jpet.108.136457. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad S.S., Najmi A.K., Kaundal M., Akhtar M. Gastroprotective effect of thymoquinone on water immersion restraint stress induced ulceration in rats. Drug Res (Stuttg) 2017 Jun;67(6):366–372. doi: 10.1055/s-0043-103574. [DOI] [PubMed] [Google Scholar]
- 8.Motegi S.I., Sekiguchi A., Uchiyama A., Uehara A., Fujiwara C., Yamazaki S. Protective effect of mesenchymal stem cells on the pressure ulcer formation by the regulation of oxidative and endoplasmic reticulum stress. Sci Rep. 2017 Dec 7;7(1):17186. doi: 10.1038/s41598-017-17630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choung R.S., Talley N.J. Epidemiology and clinical presentation of stress-related peptic damage and chronic peptic ulcer. Curr Mol Med. 2008 Jun;8(4):253–257. doi: 10.2174/156652408784533823. [DOI] [PubMed] [Google Scholar]
- 10.Uramoto H., Ohno T., Ishihara T. Gastric mucosal protection induced by restraint and water-immersion stress in rats. Jpn J Pharmacol. 1990 Nov;54(3):287–298. doi: 10.1254/jjp.54.287. [DOI] [PubMed] [Google Scholar]
- 11.Das D., Bandyopadhyay D., Bhattacharjee M., Banerjee R.K. Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic Biol Med. 1997;23(1):8–18. doi: 10.1016/s0891-5849(96)00547-3. [DOI] [PubMed] [Google Scholar]
- 12.da Rosa R.L., de Almeida C.L., Somensi L.B., Boeing T., Mariano L.N.B., de Medeiros Amorim K.C. Chrysophyllum cainito (apple-star): a fruit with gastroprotective activity in experimental ulcer models. Inflammopharmacology. 2017 Dec 8 doi: 10.1007/s10787-017-0427-z. PMID: 29222687. [DOI] [PubMed] [Google Scholar]
- 13.Dacha S., Razvi M., Massaad J., Cai Q., Wehbi M. Hypergastrinemia. Gastroenterol Rep (Oxf) 2015 Aug;3(3):201–208. doi: 10.1093/gastro/gov004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheen E., Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011 Apr;56(4):931–950. doi: 10.1007/s10620-010-1560-3. [DOI] [PubMed] [Google Scholar]
- 15.Olden K.W. The use of antidepressants in functional gastrointestinal disorders: new uses for old drugs. CNS Spectr. 2005 Nov;10(11):891–896. doi: 10.1017/s1092852900019866. [DOI] [PubMed] [Google Scholar]
- 16.Shrivastava R.K., Siegal H., Lawlor R., Shah B.K., Dayican G. Doxepin therapy for duodenal ulcer: a controlled trial in patients who failed to respond to cimetidine. Clin Ther. 1985;7(3):319–326. [PubMed] [Google Scholar]
- 17.Lewis J.D., Strom B.L., Localio A.R., Metz D.C., Farrar J.T., Weinrieb R.M. Moderate and high affinity serotonin reuptake inhibitors increase the risk of upper gastrointestinal toxicity. Pharmacoepidemiol Drug Saf. 2008 Apr;17(4):328–335. doi: 10.1002/pds.1546. [DOI] [PubMed] [Google Scholar]
- 18.de Abajo F.J., Rodriguez L.A., Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ. 1999 Oct 23;319(7217):1106–1109. doi: 10.1136/bmj.319.7217.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilici M., Ozturk C., Dursun H., Albayrak F., Saglam M.B., Uyanik A. Protective effect of mirtazapine on indomethacin-induced ulcer in rats and its relationship with oxidant and antioxidant parameters. Dig Dis Sci. 2009 Sep;54(9):1868–1875. doi: 10.1007/s10620-008-0560-z. [DOI] [PubMed] [Google Scholar]
- 20.Utkan T., Ulak G., Yildiran H.G., Yardimoglu M., Gacar M.N. Investigation on the mechanism involved in the effects of agmatine on ethanol-induced gastric mucosal injury in rats. Life Sci. 2000 Mar 24;66(18):1705–1711. doi: 10.1016/s0024-3205(00)00493-8. [DOI] [PubMed] [Google Scholar]
- 21.Dursun H., Bilici M., Albayrak F., Ozturk C., Saglam M.B., Alp H.H. Antiulcer activity of fluvoxamine in rats and its effect on oxidant and antioxidant parameters in stomach tissue. BMC Gastroenterol. 2009 May 20;9:36. doi: 10.1186/1471-230X-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glavin G.B., Alvarez I., Colombo M., Farre A.J. Effects of a novel 5-HT1A receptor agonist, E4424, on gastric adherent mucus levels following restraint stress in rats. Dig Dis Sci. 1995 Nov;40(11):2317–2320. doi: 10.1007/BF02063231. [DOI] [PubMed] [Google Scholar]
- 23.Burka J.F., Blair R.M., Hogan J.E. Characterization of the muscarinic and serotoninergic receptors of the intestine of the rainbow trout (Salmo gairdneri) Can J Physiol Pharmacol. 1989 May;67(5):477–482. doi: 10.1139/y89-076. [DOI] [PubMed] [Google Scholar]
- 24.Tack J., Janssen P., Masaoka T., Farre R., Van O.L. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012 Nov;10(11):1239–1245. doi: 10.1016/j.cgh.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 25.Spina E., de L.J. Clinically relevant interactions between newer antidepressants and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2014 May;10(5):721–746. doi: 10.1517/17425255.2014.885504. [DOI] [PubMed] [Google Scholar]
- 26.Glavin G.B., Pare W.P., Sandbak T., Bakke H.K., Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1994;18(2):223–249. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 27.Schlezinger J.J., White R.D., Stegeman J.J. Oxidative inactivation of cytochrome P-450 1A (CYP1A) stimulated by 3,3',4,4'-tetrachlorobiphenyl: production of reactive oxygen by vertebrate CYP1As. Mol Pharmacol. 1999 Sep;56(3):588–597. doi: 10.1124/mol.56.3.588. [DOI] [PubMed] [Google Scholar]
- 28.Brodie D.A., Hooke K.F. The effect of vasoactive agents on stress-induced gastric hemorrhage in the rat. Digestion. 1971;4(4):193–204. doi: 10.1159/000197120. [DOI] [PubMed] [Google Scholar]
- 29.Palle S., Kanakalatha A., Kavitha C.N. Gastroprotective and antiulcer effects of Celastrus paniculatus seed oil against several gastric ulcer models in rats. J Diet Suppl. 2017 Aug 17:1–13. doi: 10.1080/19390211.2017.1349231. [DOI] [PubMed] [Google Scholar]
- 30.Leong A.S., Milios J. Rapid immunoperoxidase staining of lymphocyte antigens using microwave irradiation. J Pathol. 1986 Feb;148(2):183–187. doi: 10.1002/path.1711480209. [DOI] [PubMed] [Google Scholar]
- 31.Greenwell A., Foley J.F., Maronpot R.R. Detecting proliferating cell nuclear antigen in archival rodent tissues. Environ Health Perspect. 1993 Dec;101(Suppl. 5):207–209. doi: 10.1289/ehp.93101s5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 33.Moron M.S., DePierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979 Jan 4;582(1):67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 34.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 35.Godara R., Katoch R., Yadav A., Ahanger R.R., Bhutyal A.D., Verma P.K. In vitro acaricidal activity of ethanolic and aqueous floral extracts of Calendula officinalis against synthetic pyrethroid resistant Rhipicephalus (Boophilus) microplus. Exp Appl Acarol. 2015 Sep;67(1):147–157. doi: 10.1007/s10493-015-9929-9. [DOI] [PubMed] [Google Scholar]
- 36.Cho C.H., Pfeiffer C.J. Gastrointestinal ulceration in the Guinea pig in response to dimaprit, histamine, and H1- and H2-blocking agents. Dig Dis Sci. 1981 Apr;26(4):306–311. doi: 10.1007/BF01308370. [DOI] [PubMed] [Google Scholar]
- 37.Cho C.H., Ogle C.W. Cholinergic-mediated gastric mast cell degranulation with subsequent histamine H1-and H2-receptor activation in stress ulceration in rats. Eur J Pharmacol. 1979 Apr 1;55(1):23–33. doi: 10.1016/0014-2999(79)90144-4. [DOI] [PubMed] [Google Scholar]
- 38.Rao C.V., Sairam K., Goel R.K. Experimental evaluation of Bocopa monniera on rat gastric ulceration and secretion. Indian J Physiol Pharmacol. 2000 Oct;44(4):435–441. [PubMed] [Google Scholar]
- 39.Guo S., Gao Q., Jiao Q., Hao W., Gao X., Cao J.M. Gastric mucosal damage in water immersion stress: mechanism and prevention with GHRP-6. World J Gastroenterol. 2012 Jun 28;18(24):3145–3155. doi: 10.3748/wjg.v18.i24.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guth P.H., Kozbur X. Pathogenesis of gastric microcirculatory and mast cell changes in restraint stress. Am J Dig Dis. 1968 Jun;13(6):530–535. doi: 10.1007/BF02233065. [DOI] [PubMed] [Google Scholar]
- 41.Mancinelli S., de la Fuente G., Manriquez V., Aracena M., Munoz R., Mancinelli S. The etiopathogenesis of the acute stress ulcer. The role of oxygen free radicals. Rev Med Chil. 1990 Sep;118(9):965–970. [PubMed] [Google Scholar]
- 42.Brodie D.A., Marshall R.W., Moreno O.M. Effect of restraint on gastric acidity in the rat. Am J Physiol. 1962 Apr;202:812–814. doi: 10.1152/ajplegacy.1962.202.4.812. [DOI] [PubMed] [Google Scholar]
- 43.Kitagawa H., Fujiwara M., Osumi Y. Effects of water-immersion stress on gastric secretion and mucosal blood flow in rats. Gastroenterology. 1979 Aug;77(2):298–302. [PubMed] [Google Scholar]
- 44.Watanabe K. Some pharmacological factors involved in formation and prevention of stress ulcer in rats. Chem Pharm Bull (Tokyo) 1966 Feb;14(2):101–107. doi: 10.1248/cpb.14.101. [DOI] [PubMed] [Google Scholar]
- 45.Hano J., Bugajski J., Wantuch C. The effect of drugs interfering with biogenic amines metabolism on gastric secretion and reserpine-ulcers development in rats. Pol J Pharmacol Pharm. 1978 Jul;30(4):501–511. [PubMed] [Google Scholar]
- 46.Takeuchi K., Ueshima K., Hironaka Y., Fujioka Y., Matsumoto J., Okabe S. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Relation to gastric hypermotility. Digestion. 1991;49(3):175–184. doi: 10.1159/000200718. [DOI] [PubMed] [Google Scholar]
- 47.Miura T., Muraoka S., Fujimoto Y. Lipid peroxidation induced by indomethacin with horseradish peroxidase and hydrogen peroxide: involvement of indomethacin radicals. Biochem Pharmacol. 2002 Jun 1;63(11):2069–2074. doi: 10.1016/s0006-2952(02)00995-4. [DOI] [PubMed] [Google Scholar]
- 48.Coskun T., Yegen B.C., Alican I., Peker O., Kurtel H. Cold restraint stress-induced gastric mucosal dysfunction. Role of nitric oxide. Dig Dis Sci. 1996 May;41(5):956–963. doi: 10.1007/BF02091537. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi K., Yasuhiro T., Asada Y., Sugawa Y. Role of nitric oxide in pathogenesis of aspirin-induced gastric mucosal damage in rats. Digestion. 1998 Jul;59(4):298–307. doi: 10.1159/000007506. [DOI] [PubMed] [Google Scholar]
- 50.Theoharides T.C., Bondy P.K., Tsakalos N.D., Askenase P.W. Differential release of serotonin and histamine from mast cells. Nature. 1982 May 20;297(5863):229–231. doi: 10.1038/297229a0. [DOI] [PubMed] [Google Scholar]
- 51.Tarnawski A., Szabo I.L., Husain S.S., Soreghan B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J Physiol Paris. 2001 Jan;95(1–6):337–344. doi: 10.1016/s0928-4257(01)00046-8. [DOI] [PubMed] [Google Scholar]
- 52.Hajrezaie M., Salehen N., Karimian H., Zahedifard M., Shams K., Al B.R. Biochanin a gastroprotective effects in ethanol-induced gastric mucosal ulceration in rats. PLoS One. 2015;10(3):e0121529. doi: 10.1371/journal.pone.0121529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Polo C.M., Moraes T.M., Pellizzon C.H., Marques M.O., Rocha L.R., Hiruma-Lima C.A. Gastric ulcers in middle-aged rats: the healing effect of essential oil from Citrus aurantium L. (Rutaceae) Evid Based Complement Alternat Med. 2012;2012:509451. doi: 10.1155/2012/509451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jesus N.Z., Falcao H.S., Lima G.R., Caldas Filho M.R., Sales I.R., Gomes I.F. Hyptis suaveolens (L.) Poit (Lamiaceae), a medicinal plant protects the stomach against several gastric ulcer models. J Ethnopharmacol. 2013 Dec 12;150(3):982–988. doi: 10.1016/j.jep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 56.Meister A. New aspects of glutathione biochemistry and transport: selective alteration of glutathione metabolism. Fed Proc. 1984 Dec;43(15):3031–3042. [PubMed] [Google Scholar]
- 57.Chattopadhyay I., Bandyopadhyay U., Biswas K., Maity P., Banerjee R.K. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006 Apr 15;40(8):1397–1408. doi: 10.1016/j.freeradbiomed.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 58.Townsend D.M., Tew K.D., Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003 May;57(3–4):145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houghton P.J., Zarka R., de las H.B., Hoult J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995 Feb;61(1):33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 60.Peric I., Stanisavljevic A., Gass P., Filipovic D. Fluoxetine reverses behavior changes in socially isolated rats: role of the hippocampal GSH-dependent defense system and proinflammatory cytokines. Eur Arch Psychiatry Clin Neurosci. 2017 Dec;267(8):737–749. doi: 10.1007/s00406-017-0807-9. [DOI] [PubMed] [Google Scholar]
- 61.Chung E.S., Chung Y.C., Bok E., Baik H.H., Park E.S., Park J.Y. Fluoxetine prevents LPS-induced degeneration of nigral dopaminergic neurons by inhibiting microglia-mediated oxidative stress. Brain Res. 2010 Dec 2;1363:143–150. doi: 10.1016/j.brainres.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 62.Mendez-David I., Tritschler L., Ali Z.E., Damiens M.H., Pallardy M., David D.J. Nrf2-signaling and BDNF: a new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci Lett. 2015 Jun 15;597:121–126. doi: 10.1016/j.neulet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 63.Zafir A., Ara A., Banu N. Invivo antioxidant status: a putative target of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Mar 17;33(2):220–228. doi: 10.1016/j.pnpbp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Huether G., Schuff-Werner P. Platelet serotonin acts as a locally releasable antioxidant. Adv Exp Med Biol. 1996;398:299–306. doi: 10.1007/978-1-4613-0381-7_47. [DOI] [PubMed] [Google Scholar]
- 65.Ries R.K., Gilbert D.A., Katon W. Tricyclic antidepressant therapy for peptic ulcer disease. Arch Intern Med. 1984 Mar;144(3):566–569. [PubMed] [Google Scholar]
- 66.Mangla J.C., Pereira M. Tricyclic antidepressants in the treatment of peptic ulcer disease. Arch Intern Med. 1982 Feb;142(2):273–275. [PubMed] [Google Scholar]
- 67.Shrivastava R.K., Siegel H. The role of tricyclics and benzodiazepine compounds in the treatment of irritable gut syndrome and peptic ulcer disease. Psychopharmacol Bull. 1984;20(4):616–621. [PubMed] [Google Scholar]
- 68.Haggerty J.J., Jr., Drossman D.A. Use of psychotropic drugs in patients with peptic ulcer. Psychosomatics. 1985 Apr;26(4):277–284. doi: 10.1016/S0033-3182(85)72859-9. [DOI] [PubMed] [Google Scholar]