Fig. 3.
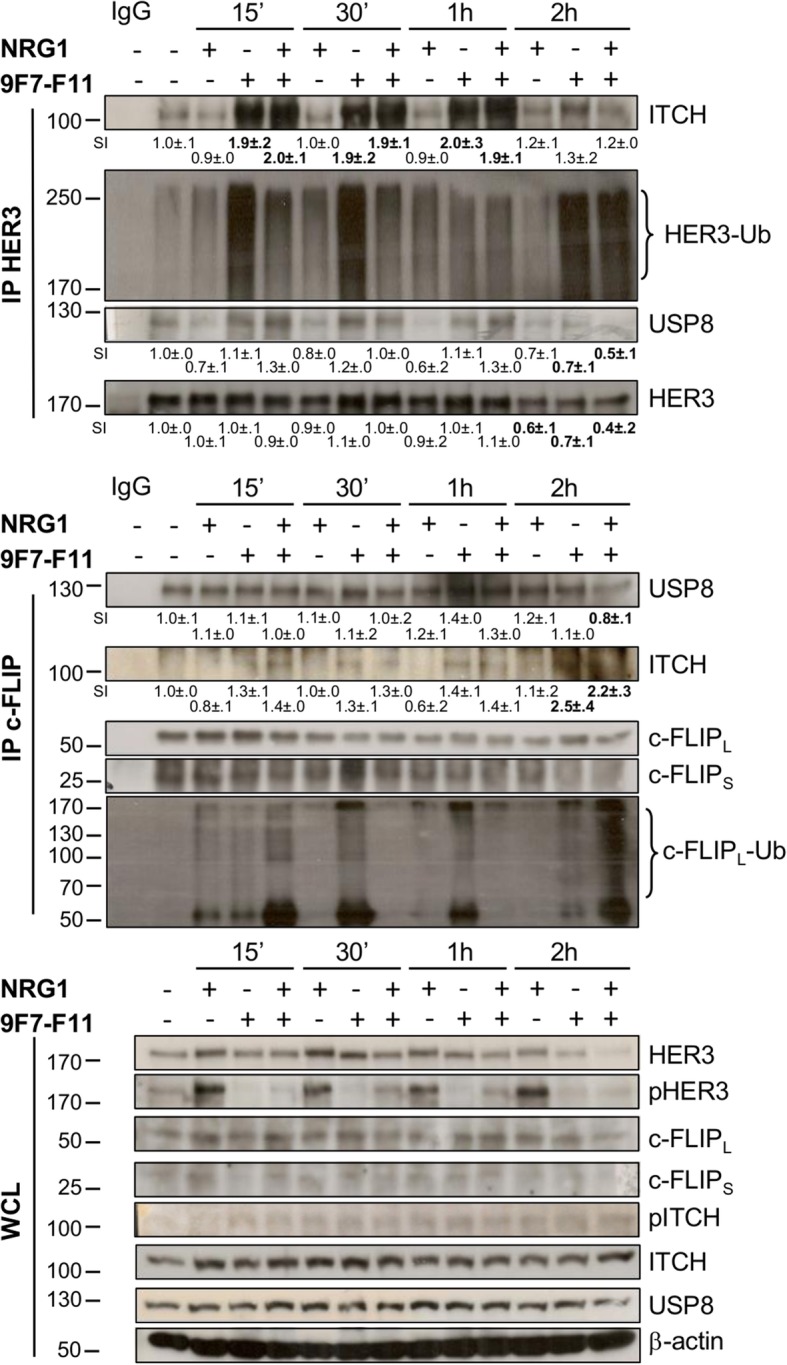
USP8-regulated ITCH interaction with c-FLIP mediates 9F7-F11-induced c-FLIP ubiquitination. BxPC3 cells were incubated with NRG1 or/and 9F7-F11 for various times. After cell lysis in CHAPS buffer, 2 mg of total protein extracts were co-immunoprecipitated with the anti-HER3 antibody 2F12 (Millipore) against HER3 C-terminal tail. Then, the first soluble supernatant was co-immunoprecipitated with the rabbit anti-c-FLIP polyclonal antibody H-202 (Santa Cruz Biotechnology) that targets both c-FLIPL and c-FLIPS. The presence of ITCH and USP8 in the two immunoprecipitates was assessed by western blotting. HER3 and c-FLIP ubiquitination status were assessed using the anti-K48 ubiquitin antibody. Whole cell lysates (WCL) were analyzed using the appropriate antibodies. Quantification of signal intensity (SI) with ImageJ software is indicated below the images, in comparison to SI = 1.0 ± .0 for untreated control. Significant increase or decrease of the densitometry, compared to control, is indicated in bold. β-actin was evaluated as loading control
