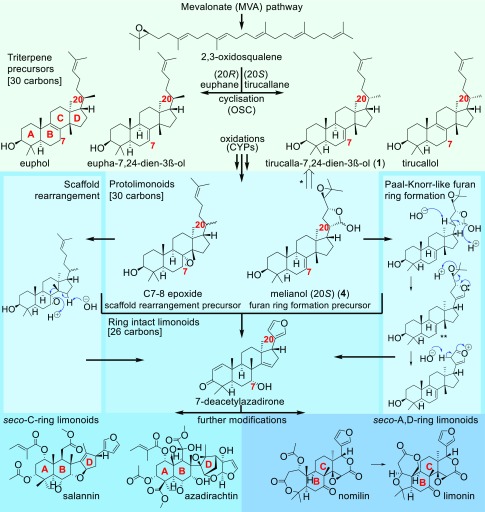
Fig. 1.
Hypothetical route of limonoid biosynthesis. Predictions of the major biosynthetic steps required for the biosynthesis of limonoids. The triterpene precursor 2,3-oxidosqualene is proposed to be cyclized to an unconfirmed tetracyclic triterpene scaffold. The structure of ring-intact limonoids implicates a tetracyclic triterpene precursor of either the euphane (20R) or tirucallane (20S) type. Retrosynthetic discrimination between these 2 side chains is impossible based on limonoid structures, because the formation of the furan ring eradicates any remnants of the precursor’s C20 stereochemistry. However, predictions can be made based on the immediate precursors of limonoids (protolimonoids); for instance, the C20 carbon of melianol (4) has been assigned (although not yet confirmed by X-ray crystallography) as the S configuration which implies a tirucallane precursor (60). Further, the C7-8 alkene of certain protolimonoids suggests the most likely triterpene precursor is in fact tirucalla-7,24-dien-3β-ol (1), as indicated by the retrosynthetic arrow (*), rather than tirucallol itself. Biosynthesis of limonoids from triterpene scaffolds is predicted to occur through protolimonoid structures such as melianol (4) and requires 2 major biosynthetic steps: scaffold rearrangements and furan ring formation accompanied by loss of 4 carbons. Scaffold rearrangement is proposed to be initiated by epoxidation of the C7 double bond (C7-8 epoxide) and furan ring formation could feasibly be initiated through oxidation and cyclization of the C20 tail (melianol) (4). The diversity of isolated protolimonoid structures has led to different predictions of the order of these 2 events (2, 61). The hemiacetal side chains of isolated protolimonoids such as melianol (4) suggest a Paal-Knorr-like (51, 52) route to the furan ring of limonoids. Isolation of nimbocinone from A. indica (SI Appendix, Fig. S1) (62), a feasible degradation product of this route (**), further supports conversion of protolimonoids to ring-intact limonoids through this mechanism. The ring-intact 7-deacetylazadirone has been isolated from both Meliaceae (63) and Rutaceae (64) species. Numerous further chemical transformations are required for the formation of seco-ring limonoid derivatives. In the Rutaceae, radioactive [14C]-labeling experiments in C. limon (lemon) have helped to delineate the late stages of the pathway, proving that nomilin can be biosynthesised in the stem and converted into other limonoids such as limonin and obacunone (SI Appendix, Fig. S1) elsewhere in the plant (65–67).