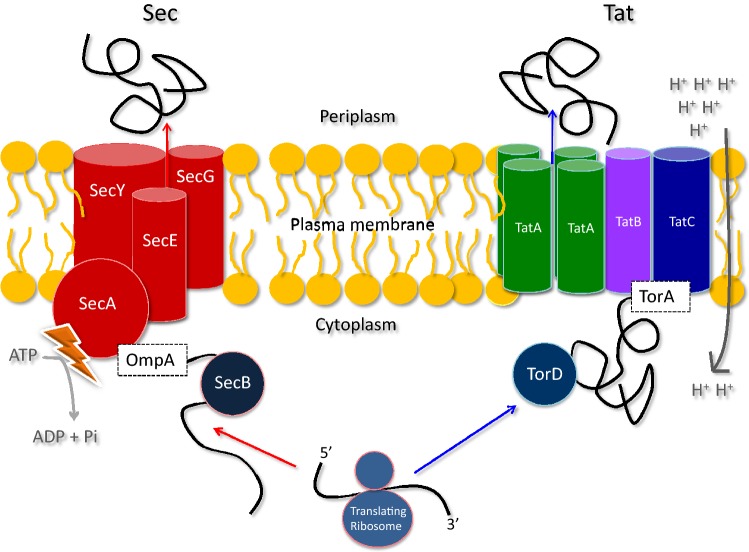
Fig. 1.
The Sec- and Tat-dependent protein transport pathways. The Sec pathway is the dominant pathway for protein export from the bacterial cytoplasm. It accepts and translocates cargo proteins across the plasma membrane in a loosely folded or unfolded state, here exemplified with the precursor of the outer membrane protein A of E. coli (OmpA). Targeting and folding control of the cargo protein is supported by cytoplasmic targeting factors, such as SecB. The Sec machinery itself is composed of the SecYEG channel and the translocation ATPase SecA, which converts chemical energy in the form of ATP into a driving force that pushes the cargo protein through the membrane. Additionally, translocation may be powered by the transmembrane proton gradient. At the trans-side of the membrane, the translocated protein folds into its active and protease-resistant final conformation. In contrast to the Sec pathway, the Tat pathway transports fully folded cofactor-containing proteins across the membrane, here exemplified with the precursor of the Tat cargo TorA. Cofactor insertion and folding may be aided by Redox Enzyme Maturation Proteins (REMPS), such as TorD in the case of TorA. The Tat translocase may consist of the three components TatA, TatB and TatC (E. coli), or of TatA and TatC components only (B. subtilis). Protein transport via Tat is powered by the transmembrane proton-motive force