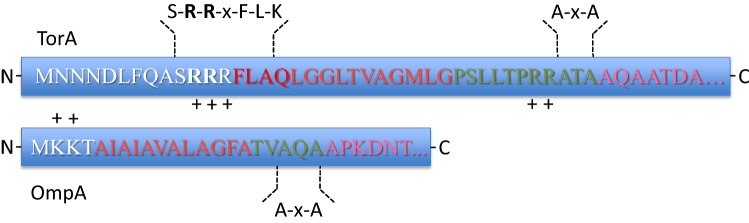
Fig. 2.
Sec- and Tat-specific signal peptides. N-terminal signal peptides direct proteins to the Sec and Tat translocases in the membrane. They have a conserved tripartite structure, consisting of a positively charged N-region (indicated by ‘white residues’ in one-letter code), a hydrophobic H-region (red) and a C-region (green) that contains the Ala-X-Ala recognition site for signal peptidase. Cleavage by signal peptidase, C-terminally from the Ala-X-Ala sequence, liberates the mature protein (pink) from the membrane. Twin-arginine signal peptides, as exemplified by the TorA signal peptide, contain the canonical twin-arginine motif at the interface of the N- and C-regions. Their H-region is longer and less hydrophobic than that of Sec-type signal peptides, and N-terminally of the C-region there are often positively charged residues that serve in Sec-avoidance. Notably, Sec-type signal peptides, here exemplified by the OmpA signal peptide, are usually much shorter than twin-arginine signal peptides