Abstract
Background:
Curcumin and resveratrol are two polyphenolic compounds extensively investigated for their medicinal effects on inflammatory signaling. However, there is a paucity of information on the Adenosine-3′, 5′-cyclic monophosphate (cAMP) kinetics following administration of curcumin and resveratrol in biological systems. In this study, kinetic modulation of cAMP as a target detection messenger in pro-inflammatory pathways was assessed by co-administration of curcumin and resveratrol using a cellular sensor model.
Methods:
To evaluate their putative activity, curcumin and resveratrol compounds were administered alone or in combination on the media culture of cAMP EPAC (exchange protein directly activated by cAMP) bioluminescence resonance energy transfer (BRET) biosensor. The study was performed at the following two centers at Tehran University of Medical Sciences (TUMS): 1- Biotechnology Research Center, and, 2- Endocrinology and Metabolism Research Institute (EMRI) in 2017. Time course kinetic of cAMP response signals were plotted. Forskolin and IBMX were used to stabilize the cAMP signals.
Results:
When we treated HEK-293T biosensor cells at 10uM concentration, curcumin and resveratrol upregulated cAMP signaling. Co-administration of resveratrol and curcumin revealed an augmented cAMP level, as compared to treatments with the compounds alone.
Conclusion:
Co-administration of curcumin and resveratrol leverage cAMP kinetic response in a time-course manner. The presented methodology can be readily adopted for drug development and novel biopharmaceutical functional analyses.
Keywords: cAMP, Cellular biosensor, Curcumin, Inflammation, Resveratrol
Introduction
Inflammation underlies numerous pathological processes that trigger such chronic human diseases as diabetes. Recent studies have shed light on our understanding of inflammatory responses and involved molecules (1–3). Cyclic adenosine monophosphate (cAMP) is a derivative of adenosine triphosphate (ATP) which used for intracellular signal transduction in many different organisms. The importance of the host cAMP axis in regulating inflammatory response encourages searching for modulating components. Curcumin (CUR), the essential constituent of turmeric (Curcuma langa L) has long been reported as having multiple health benefits in diabetes, cancers, cardiovascular and autoimmune diseases (4, 5). The therapeutic potentials of this natural polylyphenol agent have been extensively advocated due to its inherent pharmacological safety and efficacy through the suppression of numerous cell-signaling pathways. In an experimental antioxidant model, curcumin has been documented to improve STZ-induced hyperglycemia/glucose tolerance, to ameliorate the damage of pancreatic islets, to prevent type 2 Diabetes (T2D), and to inverse hypoinsulinemia (6, 7). However, despite its health benefits, the molecular targets and mechanisms of action remain abstruse, owing in part to the lack of both controllable bioavailability and kinetic detectability.
Resveratrol (RES; 3, 40, 5-trihydroxystilbene) is a polyphenolic compound found in grapes and other fruits which modulates the transcription factor NF-ϰB (8). Attributable health values for RES have been documented so far. Examples include its chemopreventive mechanism to elevate cAMP release in human breast cancer cells (9) and to protect cardiovascular disease through increased cGMP production in coronary arterial smooth muscle cells (10). Moreover, RES has been shown to possess therapeutic potential in the fight against obesity and T2DM (11). The extent of beneficial effects of RES has also been tested in a number of diabetic models including streptozotocin (STZ), nicotinamide/STZ, and long-term high-fat diets (12–14). RES supplementation improved glycemic control, and insulin sensitivity, and reduced oxidative stress in T2DM patients (15–18).
Bioluminescence resonance energy transfer (BRET) is a naturally occurring phenomenon in which a non-radiative transfer of energy from a donor like an excited luminescent enzyme or substrate to an acceptor occurs (19). In many BRET studies published to date, the donors were variants of the enzyme, Renilla reniformis luciferase (Rluc) (20, 21). Degradation of a luminescent chemical substrate, coelenterazine, by Rluc excites the acceptor fluorophore. The light emitting acceptors are variants of green fluorescent proteins.
In the present study, we applied a genetically cell biosensor, which measures intracellular cAMP concentrations in cytosol of living mammalian cells to assess the co-administration of curcumin and resveratrol influence cAMP kinetic response in a time course kinetic manner.
Materials and Methods
The study was approved by the Research Ethics Committee of the Tehran University of Medical Sciences (code 25336). The study was performed at the following two centers at Tehran University of Medical Sciences (TUMS): 1- Biotechnology Research Center and, 2- Endocrinology and Metabolism Research Institute (EMRI) in 2017.
Reagents
RES was purchased from Tocris Bioscience (Minneapolis, MN, USA). CUR, 3-isobutyl-1-methylxanthine (IBMX), and all other materials such as forskolin, coelenterazine and 3-isobutyl-1-methylxanthine (IBMX) were fine grade and obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell Culture
The wild type HEK-293T cells were purchased from National Cell Bank of Pasture Institute of Iran, Tehran, Iran. The cell line maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and antibiotics. Following overnight incubation at 37 °C, cells were collected, plated in culture flask, and allowed to reach confluency at 37 °C in a humidified atmosphere of 5% CO2 the day before the transfection using trypsin-EDTA.
The BRET-based cyclic AMP biosensor
The EPAC cAMP biosensor relies on BRET which generated by modification of the ICUE2 cAMP FRET biosensor (22). The sensor consists of an N-terminal truncated variant of the EPAC tagged with a donor (Renilla Luciferase, Rluc) and a yellow fluorescent protein variant (YFP) attached at the N and C termini, respectively. For BRET studies, the HEK-293T cells were transfected with 3 ug of BRET biosensor EPAC construct cDNA in 1 ml of calcium-phosphate transfection solution (Sigma-Aldrich, USA) and cells which express the EPAC sensor were selected.
CUR and RES treatments and BRET screening kinetic assays
HEK-293T cells permanently transfected with the EPAC sensor were split into 96-well plates at 15 to 20 × 104 cells per well. After washing with PBS on the following day, PBS (containing calcium and magnesium salt) and coelenterazine solution were added to each well. After 10 min incubation, vehicle, curcumin (10 uM), resveratrol (10 uM) or both were added. The plate was then placed into a Mithras LB940 instrument (Berthold Technologies, Bad Wildbad, Germany) that allowed the sequential integration of the luminescent signals detected in the 465 to 505 nm and 505 to 555 nm windows using filters with the appropriate band pass and by using MicroWin 2000 software (Berthold Technologies). The BRET signal is determined by calculating the ratio of the light emitted at 505 to 555 nm to the light emitted at 465 to 505 nm.
Statistical analysis
Each experiment was performed three times and normally distributed data are presented as means ± SEM. The differences in cell luminescent were determined using the Student’s t-test by the statistical software SPSS 20 (Chicago, IL, USA). P value <0.05 was considered significant.
Results
EPAC-transfected cells for measuring cAMP in real time
We evaluated the efficacy of our EPAC-transfected cells biosensor to produce cAMP in real time using forskolin and IBMX as cAMP inducer. Upon binding cAMP, the signal of the biosensor decreases because of a conformational change that presumably increased the distance between the Rluc donor and the yellow fluorescent protein acceptor. EPAC-transfected cells biosensor showed no statistically differences between these repeats in various time courses (F=0.714, 28; P>0.845).
Effect of CUR and RES on BRET cell sensor in real time
In order to evaluate effect of Curcumin and Resveratrol on cAMP signaling pathway, the mentioned amount of CUR and RES were added. The ratio of the light emitted for curcumin and Resveratrol in comparison with positive and negative components using EPAC-transfected cells biosensor assay is depicted in Fig. 1.
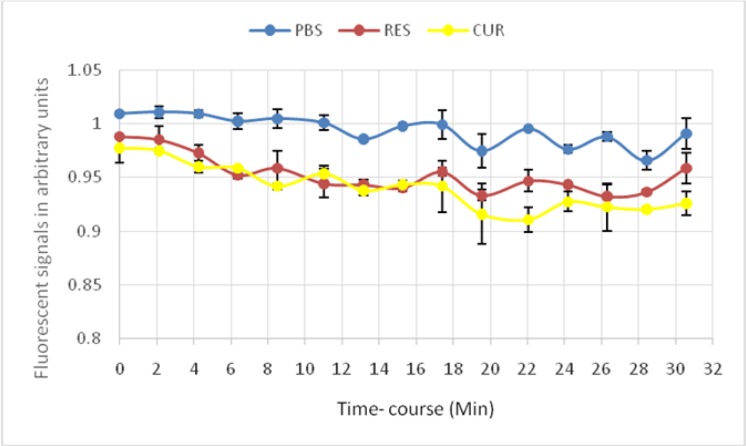
Fig. 1:
Ratio of the light emitted for CUR and RES in comparison with negative control using EPAC-transfected cells biosensor assay
Wells of duplicate rows in the 96-W test plate BRET assay were filled with equal volumes of PBS vehicle alone as blank (upper curve) or RES (mid curve) or CUR (lower curve), added by forskolin and IBMX. The significant shifting down of RES and CUR curves indicates dynamic activation of cAMP signal over time-course experiment. Data are expressed as mean± SD. From top to Bottom: 1- PBS + Forskolin+IBMX; 2-RES (10uM)+Forskolin+IBMX; 3-CUR (10uM) + Forskolin+IBMX
Although significant differences between Cur and Rev compared to control (P=0.001, P=0.001), there were no differences between CUR and RES (P=0.098) based on our statistical analysis. There were no statistically differences between these repeats in various time courses. These compounds produce a sustained reduction in the BRET signal in all time points.
Effect of CUR+REV on BRET cell sensor
The efficacy of a mixture of CUR and REV on the biosensor for measuring EPAC cAMP signaling in real time is demonstrated in Fig. 2. cAMP production was augmented statistically when a mixture of CUR & RES compare with each of them administration (P=0.001) (Figs. 3, 4).
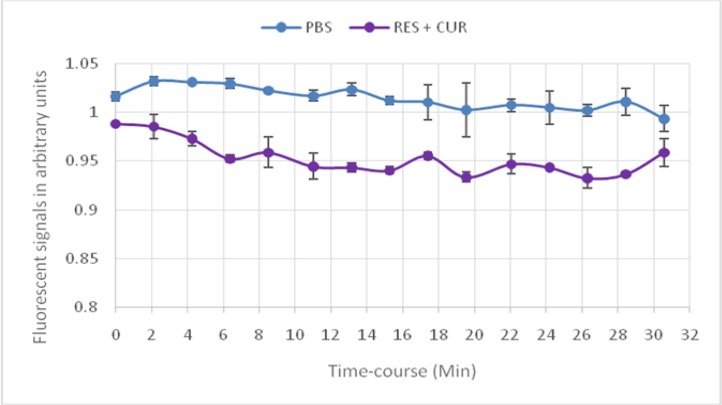
Fig.2:
Curcumin+Resveratrol in comparison with negative control using EPAC-transfected cells biosensor assay
Combination of RES and CUR (lower curve) significantly raised the cAMP time-course emission signals, as compared to the vehicle PBS blank (upper curve). Data are expressed as mean± SD. From Top to Bottom: 1-PBS+Forskolin+IBMX; 2-PBS+CUR (10uM)+RES (10uM)+ Forskolin+IBMX
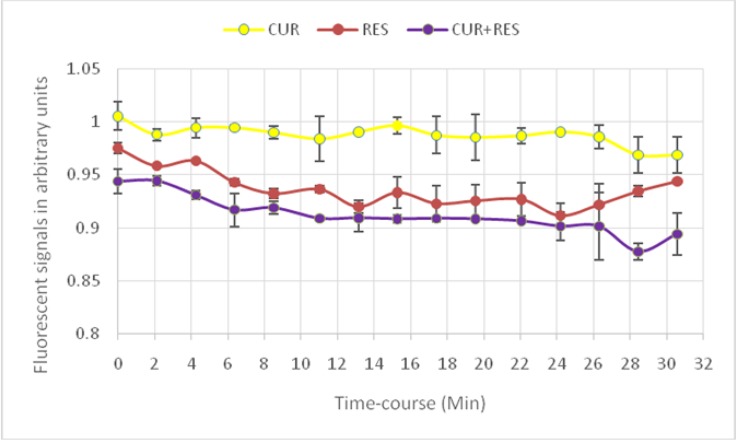
Fig. 3:
Time course of the BRET sensor response to cAMP accumulation after exposure to 10uM CUR, RES, or CUR+RES
Depiction of elevated cAMP activation signal with combined RES and CUR treatment (lower curve), as compared to RES- (mid) or CUR-alone (upper) curves. Data are expressed as mean± SD. From top to Bottom: 1- CUR (10uM)+Forskolin+IBMX; 2-RES (10uM)+Forskolin+IBMX; 3-CUR +RES(10uM) + Forskolin + IBMX
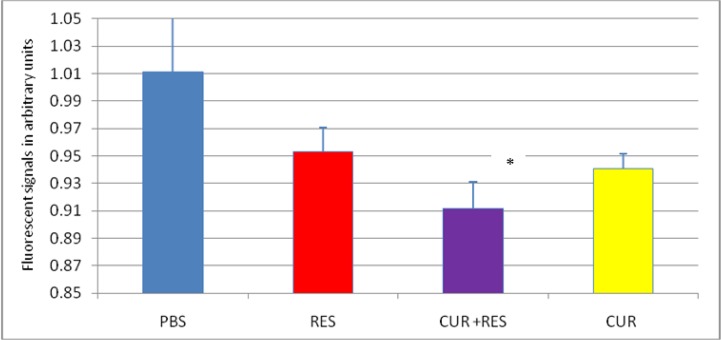
Fig. 4:
End point of the BRET sensor response to cAMP accumulation after exposure to 10uM CUR, RES, or CUR+RES
Comparative end point analysis of RES, CUR, and CUR+RES was depicted to corroborate the time-course kinetic cAMP emission BRET assays. Data are expressed as mean± SD. Significant differences are indicated by *P<0.001 compared with controls
Discussion
Subclinical inflammation is considered as one of the primary triggers for many pathological circumstances like type 2 diabetes. Although T2D is developed because of β cell dysfunction, the role of cAMP generation in these cells is not completely accepted. Experimental models of diabetes showed a reduction in cAMP induction by glucose which improved by cAMP-elevating agents (23). This plausible mechanism should be influenced by curcumin and resveratrol bioactive compounds with relatively higher safety profiles (7, 12).
The cAMP EPAC biosensor used in our studies was originally developed as a FRET biosensor (24) and later adapted for BRET applications (19). In the lack of cAMP at resting levels, there is considerable basal energy transfer between fluorophores, suggesting the donor and the acceptor remain in adjacent positioning. Whenever cAMP increases, BRET ratios decrease due to a conformational change leading to increased distance between the Rluc and YFP. The main advantage of exchange protein directly activated by cAMP is the possibility to measure the fluctuations of cAMP in Real-time. Moreover, this BRET sensor should evaluate the contribution and the kinetic of different components that modulate cAMP levels.
Based on our findings, curcumin steadily decreases the ratio of the light emitted which means that it increases the cAMP levels at the molecular level. This result in agreement that found increased tissue cAMP levels in liver and less in other adipose tissue except in interscapular subcutaneous fat (25). Because of CUR rapid metabolism in cells, we examined it in short period of time, since the effect of CUR metabolites on cAMP production should be practically faded. Recent studies in mice have revealed that curcumin modulates plasma lipid levels and mitochondrial biogenesis via increasing the level of cAMP (25, 26). Although other signaling pathways including NF-ϰB, STAT3 and Nrf2 involved in ameliorative effect of curcumin in various types of diseases, the cAMP/protein kinaseA pathway also plays a pivotal role in some of them (5).
Other examined polyphenolic compound, resveratrol, is also modulated numerous transcriptional factor like SIRT family which regulate glucose and fat metabolism; Nevertheless, the RES is not biologically effectiveness in human administration (27, 28). Here, we documented that resveratrol increased the level of cAMP. Resveratrol was capable to regulate cyclic AMP response element (CRE) in human embryonic kidney cells (29). Moreover, curcumin had no effect upon CRE-regulated transcription, which persuades its usage with other nutraceuticals such as resveratrol.
Our data showed cAMP production of a mixture of CUR and REV on the EPAC cAMP cell biosensor is augmented statistically significant compared with each of them administration. Taken together, the synergistic effect of these mention combination should be considered as a better protectiveness in cell stress conditions.
Conclusion
Dual-ingredient formulation of curcumin and resveratrol could dramatically enhance cell induction through cAMP production. This might be considered for new drug development.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This project was supported by Tehran University of Medical Sciences and Health Services, grant 25336. The EPAC biosensor constructs for transfection was a gift from Dr. Ali Salahpour from the Department of Pharmacology and Toxicology, University of Toronto. Authors would like to sincerely thanks and appreciate him for all his assistance and technical support. Special thanks to ‘Biotechnology Research Centre’ and, Endocrinology and Metabolism Research Institute (EMRI), Tehran University of Medical Sciences for supporting grant 25336.
Footnotes
Conflicts of interest
The authors have declared no conflicts of interest.
References
- 1.Caiazzo E, Ialenti A, Cicala C. (2019). The relatively selective cyclooxygenase-2 inhibitor nimesulide: What’s going on? Eur J Pharmacol, 848:105–111. [DOI] [PubMed] [Google Scholar]
- 2.Faas MM, Sáez T, de Vos P. (2017). Extracellular ATP and adenosine: The Yin and Yangin immune responses? Mol Aspects Med, 55:9–19. [DOI] [PubMed] [Google Scholar]
- 3.Shu J, Zhang F, Zhang L, et al. (2017). G protein coupled receptors signaling pathways implicate in inflammatory and immune response of rheumatoid arthritis. Inflamm Res,66(5):379–387. [DOI] [PubMed] [Google Scholar]
- 4.Mirzaei H, Shakeri A, Rashidi B, et al. (2017). Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed Pharmacother, 85:102–112. [DOI] [PubMed] [Google Scholar]
- 5.Kunnumakkara AB, Bordoloi D, Padmavathi G, et al. (2017). Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol, 174(11):1325–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Azab MF, Attia FM, El-Mowafy AM. (2011). Novel role of curcumin combined with bone marrow transplantation in reversing experimental diabetes: Effects on pancreatic islet regeneration, oxidative stress, and inflammatory cytokines. Eur J Pharmacol, 658(1): 41–8. [DOI] [PubMed] [Google Scholar]
- 7.Thota RN, Acharya SH, Abbott KA, et al. (2016). Curcumin and long-chain Omega-3 polyunsaturated fatty acids for Prevention of type 2 Diabetes (COP-D): study protocol for a randomised controlled trial. Trials, 17(1):565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Gerevini GT, Repossi G, Dain A, et al. (2016). Beneficial action of resveratrol: How and why? Nutrition, 32(2): 174–8. [DOI] [PubMed] [Google Scholar]
- 9.El-Mowafy AM, Alkhalaf M. (2003). Resveratrol activates adenylyl-cyclase in human breast cancer cells: a novel, estrogen receptor-independent cytostatic mechanism. Carcinogenesis, 24(5): 869–73. [DOI] [PubMed] [Google Scholar]
- 10.El-Mowafy AM. (2002). Resveratrol activates membrane-bound guanylyl cyclase in coronary arterial smooth muscle: a novel signaling mechanism in support of coronary protection. Biochem Biophys Res Commun, 291(5): 1218–24. [DOI] [PubMed] [Google Scholar]
- 11.Beaudeux JL, Nivet-Antoine V, Giral P. (2010). Resveratrol: a relevant pharmacological approach for the treatment of metabolic syndrome? Curr Opin Clin Nutr Metab Care, 13(6): 729–36. [DOI] [PubMed] [Google Scholar]
- 12.Kamaleddin MA. (2016). The paradoxical pro- and antiangiogenic actions of resveratrol: therapeutic applications in cancer and diabetes. Ann N Y Acad Sci, 1386(1): 3–15. [DOI] [PubMed] [Google Scholar]
- 13.Szkudelska K, Nogowski L, Szkudelski T. (2011). Resveratrol and genistein as adenosine triphosphate-depleting agents in fat cells. Metabolism, 60(5): 720–9. [DOI] [PubMed] [Google Scholar]
- 14.Fiori JL, Shin YK, Kim W, et al. (2013). Resveratrol prevents β-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes, 62 (10): 3500–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kar P, Laight D, Rooprai HK, et al. (2009). Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med, 26 (5):526–31. [DOI] [PubMed] [Google Scholar]
- 16.Brasnyó P, Molnár GA, Mohás M, et al. (2011). Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr, 106 (3): 383–9. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt JK, Thomas S, Nanjan MJ. (2012). Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res, 32 (7):537–41. [DOI] [PubMed] [Google Scholar]
- 18.Hausenblas HA, Schoulda JA, Smoliga JM. (2015). Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus--systematic review and meta-analysis. Mol Nutr Food Res, 59(1): 147–59. [DOI] [PubMed] [Google Scholar]
- 19.Barak LS, Salahpour A, Zhang X, et al. (2008). Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor. Mol Pharmacol, 74(3): 585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi H, Picard LP, Schönegge AM, et al. (2019). Bioluminescence resonance energy transfer-based imaging of protein-protein interactions in living cells. Nat Protoc, 14(4):1084–1107. [DOI] [PubMed] [Google Scholar]
- 21.Wimmer T, Schroeter E, Lorenz B, et al. (2017). Detection of the Vascular Endothelial Growth Factor with a Novel Bioluminescence Resonance Energy Transfer Pair Using a Two-Component System. Sensors (Basel), 17(1):E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Violin JD, DiPilato LM, Yildirim N, et al. (2008). Beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem, 283(5): 2949–61. [DOI] [PubMed] [Google Scholar]
- 23.Tengholm A. (2012). Cyclic AMP dynamics in the pancreatic b-cell. Ups J Med Sci, 117(4):355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiPilato LM, Cheng X, Zhang J. (2004). Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A, 101(47): 16513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zingg JM, Hasan ST, Nakagawa K, et al. (2017). Modulation of cAMP levels by high-fat diet, curcumin, and regulatory effects on CD36/FAT scavenger receptor/fatty acids transporter gene expression. Biofactors, 43(1):42–53. [DOI] [PubMed] [Google Scholar]
- 26.Ray Hamidie RD, Yamada T, Ishizawa R, et al. (2015). Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism, 64(10): 1334–47. [DOI] [PubMed] [Google Scholar]
- 27.Bruckbauer A, Zemel MB, Thorpe T, et al. (2012). Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr Metab (Lond), 9(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu S, Gao Y, Zhang Q, et al. (2016). SIRT1/3 Activation by Resveratrol Attenuates Acute Kidney Injury in a Septic Rat Model. Oxid Med Cell Longev, 2016: 7296092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiel G, Rössler OG. (2016). Resveratrol stimulates cyclic AMP response element mediated gene transcription. Mol Nutr Food Res, 60(2): 256–65. [DOI] [PubMed] [Google Scholar]