Abstract
Intravesical instillation of Bacille Calmette–Guèrin (BCG) is the standard adjuvant treatment for high-risk non muscle invasive bladder cancer (NMIBC). Since its mechanism of action is supposed to be linked to the immune system efficiency and senescence could negatively affect this efficiency, BCG efficacy in the elderly has been questioned. This study aimed to assess the impact of age on BCG efficacy and safety in patients with high-grade T1 bladder cancer (BC).
Among 123 patients with high-grade T1 BCG scheduled for BCG treatment, 82 were <75 year-old (group A) and 41 were ≥75 year-old (group B). Follow-up: urine cytology and cystoscopy every 3 months for the first 2 years, every 6 months for the third year, and then yearly. Tumor recurrence was defined as pathological evidence of disease at the bladder biopsy; tumor progression was defined as pathological shift to muscle invasive disease at the bladder biopsy or the imaging techniques showing recurrent BC and distant metastasis likely related to it.
The median follow-up was 65 months (range 11-152). Recurrence occurred in 35 patients, 19 (23.2%) in the group A and 16 (39%) in the group B. Progression occurred in 18 patients, 12 (14.6%) in the group A and 6 (14.6%) in the group B. Recurrence free rate was similar in both groups up to 2 years. The 5 years progression rate was almost the same in both groups A and B (85.9% vs 84.7%), whereas the 5 years cancer-specific survival (CSS) was 92.6% in the group A and 85.4% in the group B. Of the 18 patients with progression, 11 underwent cystectomy; 12 patients died because of their BC. Kaplan–Meier plots pointed out no difference in recurrence-free, progression-free, and CSS between the 2 groups. Adverse events were similar in the 2 groups. Only 4 (3.3%) patients, 2 (2.4%) in the group A and 2 (4.8%) in the group B, experienced mild adverse reactions compatible with treatment.
Elderly patients with high-grade T1 BC are not poorer candidates to BCG treatment, as they had similar benefit and adverse reactions than those aging ≥75 years.
Keywords: Bacille Calmette–Guèrin, non muscle invasive bladder cancer, transurethral resection of the bladder tumor
1. Introduction
Bladder cancer (BC) is the 5th most common worldwide cancer and its incidence rises with the age of the patient.[1] Increase in life expectancy therefore leads to more elderly patients seeking treatment for this disease.
Intravesical instillation of Bacille Calmette–Guèrin (BCG) is the standard adjuvant treatment for high-risk non muscle invasive bladder cancer (NMIBC) after transurethral resection of the bladder tumor (TURBT).[2] The postulated mechanism of action of intravesical BCG is based onto activation and migration of immune cells into bladder wall, exploding an antitumoral effect mediated by the interaction between antigen presenting cells and lymphocytes Th1. This leads to priming of CD8+ cytotoxic T-cells, activation of Natural Killer cells, and release of inflammatory cytokines such as Interferon [INF]-gamma, Interleukin [IL]-12 and tumor necrosis factor [TNF]-alfa.[3] The clinical response to intravesical BCG administration seems to be linked to the strength of the immune response.[4]
Several studies pointed out that in the elderly the innate and adaptive immune systems are deteriorated, a phenomenon known as immunosenescence.[4–6] Weakness of the immune system has been suggested to reduce response to BCG and increase the risk of adverse reactions.[4] Indeed, current EAU guidelines highlight that BCG is less effective in patients >70 years old.[2] This makes the choice of the intravesical adjuvant treatment in the elderly with high-risk NMIBC quite challenging.
The present study aimed to assess the impact of age on BCG efficacy and safety in a homogeneous population of patients with high-grade T1 BC who received intravesical instillations of BCG after TURBT.
2. Materials and methods
We analyzed prospectively our NMIBC database to identify patients with high-grade T1 BC[2] who received intravesical instillation of BCG. All patients underwent bladder biopsies/TUR 5-8 weeks after having completed the BCG induction cycle. Patients who responded to induction treatment underwent BCG maintenance for 12 months. Patients with incomplete clinical data were excluded.
Follow-up consisted of urine cytology and cystoscopy every 3 months for the first 2 years, every 6 months for the third year, and then yearly. Chest and abdominal computed tomography was performed at the initial diagnosis and then every year to rule out upper urinary tract or metastatic disease. Tumor recurrence was defined as pathological evidence of disease at the bladder biopsy or TURBT, whereas tumor progression was defined as the pathological shift to muscle invasive disease at the bladder biopsy or TURBT or imaging techniques demonstrating recurrent bladder cancer and distant metastasis likely related to it.
Two senior pathologists unaware of clinical data reviewed all specimens including agreement with the latest WHO Classification of Tumors of the Urinary System and Male Genital Organs[7] and the 2010 TNM staging system[8].
The study protocol had been approved by the Internal Ethical Review Board and conformed to the provisions of the Declaration of Helsinki.
2.1. Statistical analysis
Continuous data are reported as means ± standard deviations (SD) or median values as appropriate; those with normal distribution according to the Skewness and Kurtosis test were compared by Student t test for paired or unpaired data. Continuous data with a non-parametric distribution were compared by the Mann–Whitney U test for independent groups. Differences in rates were compared by the Chi-Squared test or the Fisher exact test. Univariate analysis of disease free survival (RFS), progression free survival (PFS), and cancer specific survival (CSS) was carried out using the Kaplan–Meier method, with differences among groups being tested for significance using the Log-rank test.
Univariate and multivariate analysis of probable prognostic factors for DFS, PFS, and CSS was carried out using Cox proportional hazard regression analysis. Significance was set at P < .05. Statistical analysis was carried out using the MedCalc 16.8 Software (MedCalc, Ostend, Belgium) and STATA SE 15.
3. Results
From January 2005 to January 2018, a total of 128 patients with high-grade T1 BCG were scheduled for BCG treatment. During the induction cycle, 5 patients, 4 aging <75 years and 1 aging ≥75 years, discontinued treatment due to recurrent urinary tract infections.
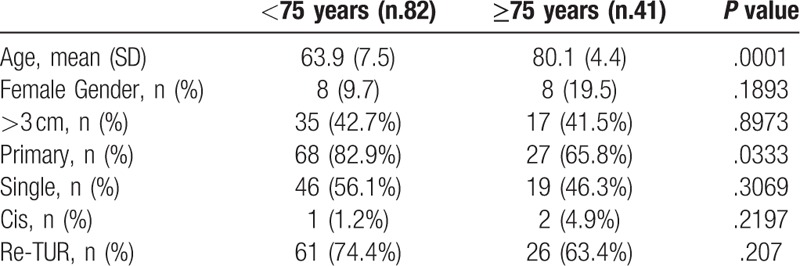
Table 1 reports the descriptive characteristics of the 123 patients who remained on BCG treatment, showing no significant difference between patients aging <75 years (group A) and those aging ≥75 years (group B).
Table 1.
Patients data according to age.
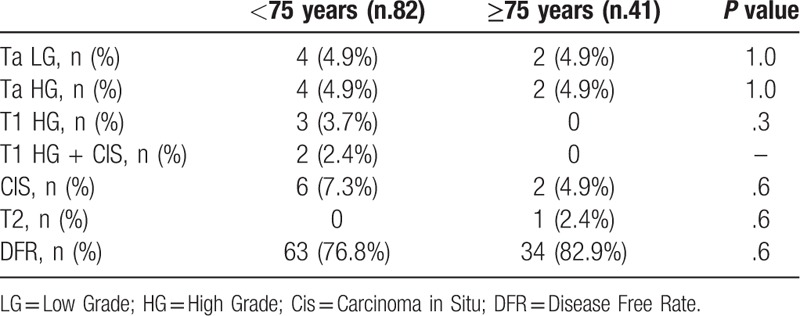
The results of the bladder biopsies/TUR after BCG induction cycle in both groups are summarized in Table 2. The patient with T2 disease and 2 patients with high-grade T1 and concomitant carcinoma in situ (CIS) underwent cystectomy, whereas 9 patients with high grade T1 BC or CIS underwent the second induction cycle. The remaining patients were scheduled for BCG maintenance.
Table 2.
Results of bladder biopsies/TUR after BCG induction cycle.
During the maintenance phase, 4 (3.3%) patients experienced adverse reactions (AR), 2 (2.4%) in the group A and 2 (4.8%) in the group B (P = .47). All AR consisted in flu-like symptoms (fever, malaise) lasting <24 hours, mild bladder pain and moderate lower urinary tract symptoms (nocturia, frequency, urgency). All reactions responded to symptomatic treatment but 1 patient aging <75 years preferred to discontinue BCG treatment.
3.1. Oncological results
The median follow-up in the 120 patients who remained on BCG treatment was 65 months (range 11–152). Recurrence occurred in 35 patients, 19 (23.2%) in the group A and 16 (39%) in the group B. Progression happened in 18 patients, 12 (14.6%) in the group A and 6 (14.6%) in the group B. Six progressions occurred after disease recurrence and consisted of local disease in 5 and local disease and liver metastases in 1 case; they were 3 (3.6%) in the group A and 3 (7.3%) in the group B. Twelve patients presented direct disease progression (9 local diseases and 3 associated to multiple pulmonary metastases); they were 9 (10.9%) in the group A and 3 (7.3%) in the group B. Of the 18 patients who progressed, 11 underwent cystectomy; 12 patients eventually died because of their BC, of those 6 in the group A and 6 in the group B, 7,5% and 15%, respectively (P = .196).
Recurrence free rate was similar in both groups up to 2 years, becoming lower in the group B afterwards; Kaplan–Meier plots however showed that such difference was not statistically significant (Fig. 1A). Most importantly, the 5 year progression rate was almost the same in the group A and B (85.9% vs. 84.7%), whereas the 5 year CSS was 92.6% in the group A and 85.4% in the group B; Kaplan–Meier plots pointed out no difference in PFS and CSS (Fig. 1B and C).
Figure 1.
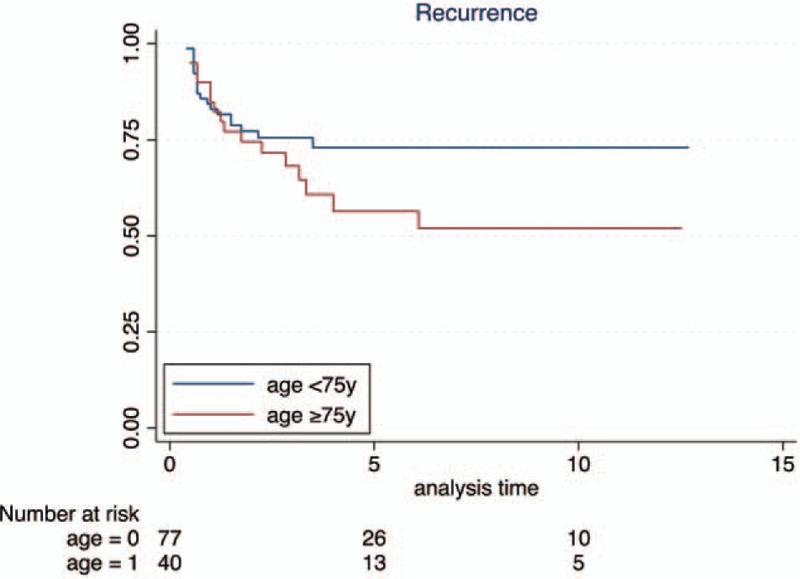
Kaplan–Meier curves for recurrence-free survival stratified by age, P value = .1754.
Univariate Cox proportional hazard regression analysis pointed out that none of tested variables predicted RFS, PFS, and CSS (Table 3), thus making multivariate analysis un-necessary ().
Table 3.
Cox univariate logistic regression analysis of potential predictors of RFS, PFS, and CSS.
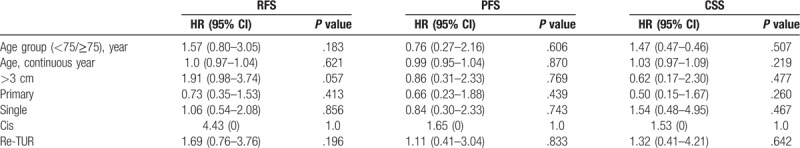
Figure 2.
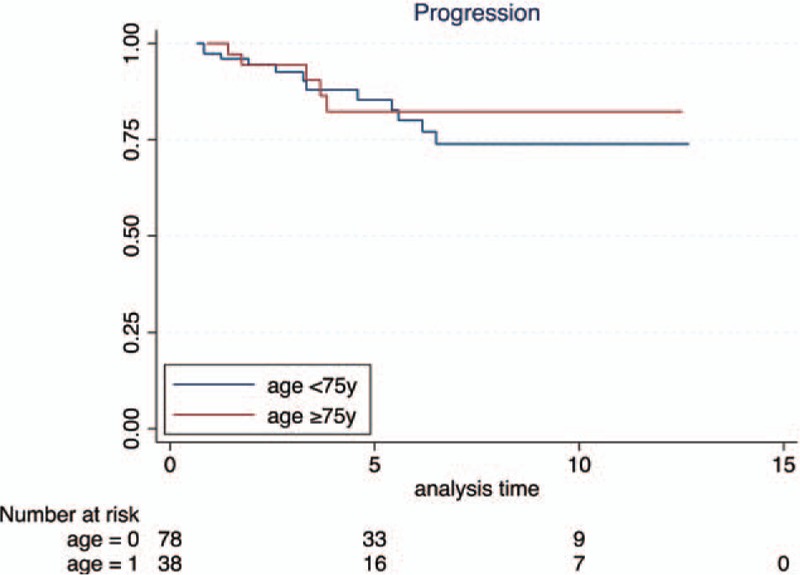
Kaplan–Meier curves for progression-free survival stratified by age, P value = .6041.
Figure 3.
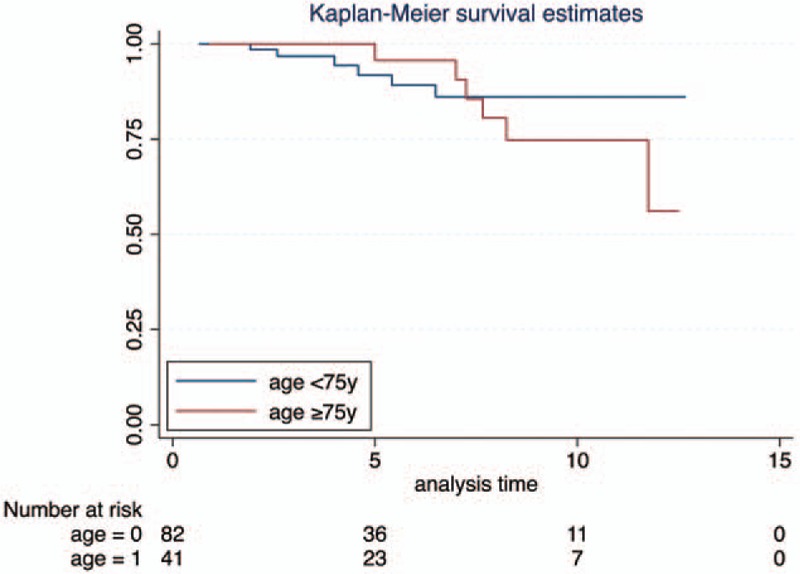
Kaplan–Meier curves for cancer-specific survival stratified by age, P value = .5045.
4. Discussion
The present study pointed out that age does not negatively affect the results of BCG treatment in terms of RFS, PFS, and CSS and does not significantly increase the risk of adverse reactions to such treatment.
The role of aging on BCG efficacy is controversial. In a large study on 805 patients, Herr et al [9] established that aging had a measurable, yet small impact on RFS but no impact on the overall outcomes (PFS and CSS) of patients with high-grade Ta or T1 BC treated with BCG. Apart from including both Ta and T1 tumors, potential study limitations could be the exclusion of patients with early failure and evaluation of BCG response only after 6 months.
Margel et al [10] compared 158 patients <75years with 80 patients ≥75years and found no difference in RFS but a statistically significant (P < .001) difference in PFS. Cox multivariate proportional hazard regression analysis demonstrated that age was the most significant independent predictor of progression (HR 2.1) followed by BCG maintenance (HR 0.8). However, this study included both low-grade and high-grade tumors, Ta, T1, and CIS. Similarly, Oddens et al [11] comparing 534 patients younger than 70 years with a cohort of 288 patients older than 70 years, reported that, at mean follow-up of 9.2 years, age did not affect RFS but patients >70 years had a worst PFS and CSS than those <70 years. However, also this study has the limitations of having included both low-grade and high-grade tumors, stage Ta and T1.
Because of the increase in life expectancy, we defined 75 years as the cut-off to conduct our analysis, more closely reflecting the current clinical practice.
Inspired by the study of Oddens et al, [11] we tried to analyze our data moving the cut-off to 70 years; in this way we had 63 patients ≤70 years and 60 patients >70 years. Therefore, for a further confirmation of our thesis, the analysis conducted by moving the cut-off showed no difference in RFS, PFS, and CSS (P = .478, .853, and .289, respectively).
A strong point of our study was having included only high-grade T1 tumors which had been blindly reviewed by two dedicated uro-pathologists as well as having controlled survival for clinico-pathological prognostic factors. It could be argued that patient characteristics could have further been evaluated by those molecular markers we previously demonstrated having a predictive role[12–19] but this seemed to be beyond the scope of this clinical study. Prospective studies would obviously provide more solid data regarding the impact of age on response to BCG treatment; in the meanwhile, a meta-analysis of patient populations homogeneous for stage, grade, clinic-pathological features, and treatment would be welcome to better define such issue.
Also the role of age on BCG toxicity is controversial. Heiner et al[20] evidenced that BCG complications were significantly (P = .001) more common in elderly patients, thus recommending to treat such patients by BCG induction only or even by “other” intravesical agents. Racioppi et al[21] found that elderly patients had more early complications but the rate of severe complications did not vary with age. However, they recommended to administering the BCG induction cycle biweekly in the elderly patients to reduce the risk of complications. Finally, a recent large study[22] on patients randomized to receive 3 years BCG maintenance reported no impact of age on treatment side-effects or discontinuation.
The present study showed no difference in treatment discontinuation between the 2 groups. Almost all discontinuations occurred during the induction cycle, somehow suggesting that patients who stand the induction cycle are likely to easily stand maintenance. ARs observed during maintenance were uncommon (3.3%) and always minor. Again age had no significant impact on their incidence. It is worth mentioning that all patients had their bladder emptied by placing a soft urethral catheter and that all were carefully instructed to drink 2 L fluids over the following 12 hours in order to better eliminate potential BCG residuals. Whether such measures could be responsible for our low rate of adverse reactions however remains to be demonstrated.
The main study limitation is the small sample size. Another limitation is having focused on patients who accepted to undergo BCG treatment, but such limitation is common to all other studies; moreover, none of our patients was shifted to any other intravesical treatment if considered unsuitable for BCG treatment. Another study limitation is its retrospective nature; again, this is common to available studies and, anyway, data were prospectively collected[9–11,20–22].
5. Conclusions
Elderly (>75 years) patients with high-grade T1 BC are not poorer candidates to BCG treatment, as they had similar benefit and adverse reactions than those aging ≤75 years.
Acknowledgments
We are grateful to Prof. Maria Michela Dota, native speaker English teacher, for her precious linguistic revision.
Author contributions
Conceptualization: Beppe Calò, Francesca Sanguedolce, Vito Mancini, Giuseppe Carrieri, Luigi Cormio.
Data curation: Beppe Calò, Francesca Sanguedolce, Francesca Fortunato, Nicola d’Altilia, Marco Chirico, Ugo Falagario, Vito Mancini, Luigi Cormio.
Formal analysis: Beppe Calò, Francesca Sanguedolce, Francesca Fortunato, Giovanni Stallone, Ugo Falagario, Vito Mancini, Luigi Cormio.
Investigation: Beppe Calò, Francesca Sanguedolce, Marco Chirico.
Methodology: Beppe Calò, Francesca Fortunato, Marco Chirico, Ugo Falagario, Luigi Cormio.
Project administration: Vito Mancini, Giuseppe Carrieri, Luigi Cormio.
Software: Francesca Fortunato, Ugo Falagario.
Supervision: Giovanni Stallone, Vito Mancini, Giuseppe Carrieri, Luigi Cormio.
Validation: Giovanni Stallone, Nicola d’Altilia, Ugo Falagario.
Visualization: Giovanni Stallone, Nicola d’Altilia, Giuseppe Carrieri.
Writing - original draft: Beppe Calò, Francesca Sanguedolce, Nicola d’Altilia, Luigi Cormio.
Writing - review & editing: Giovanni Stallone, Vito Mancini, Giuseppe Carrieri, Luigi Cormio.
Vito Mancini orcid: 0000-0003-3050-8748.
Footnotes
Abbreviations: BCG = Bacille Calmette–Guèrin, NMIBC = non muscle invasive bladder cancer, BC = bladder cancer, CSS = cancer specific survival, TURBT = transurethral resection of the bladder tumor, INF = interferon, IL = interleukine, RFS = recurrence free survival, PFS = progression free survival, AR = adverse reactions, TNF = tumor necrosis factor, SD = standard deviations, CIS = carcinoma in situ.
CB and SF equally contributed.
All the authors have nothing to declare.
The authors report no conflicts of interest
References
- [1].Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- [2].EAU Guidelines. Edn. Presented at the EAU Annual Congress Copenhagen 2018. ISBN 978-94-92671-01-1 [Google Scholar]
- [3].Kamat AM, Li R, O’Donnell MA, et al. Predicting response to intravesical Bacillus Calmette-Guérin immunotherapy: are we there yet? A systematic review. Eur Urol 2018;73:738–48. [DOI] [PubMed] [Google Scholar]
- [4].Pfister G, Savino W. Can the immune system still be efficient in the elderly? An immunological and immunoendocrine therapeutic perspective. Neuroimmunomodulation 2008;15:351–64. [DOI] [PubMed] [Google Scholar]
- [5].Reis LO, Ferreira U, Billis A, et al. Anti-angiogenic effects of the superantigen staphylococcal enterotoxin B and bacillus Calmette-Guérin immunotherapy for nonmuscle invasive bladder cancer. J Urol 2012;187:438–45. [DOI] [PubMed] [Google Scholar]
- [6].Bueno V, Sant’Anna OA, Lord JM. Ageing and myeloid-derived suppressor cells: possible involvement in immunosenescence and age-related disease. Age (Dordr) 2014;36:9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol 2016;70:93–105. [DOI] [PubMed] [Google Scholar]
- [8].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [9].Herr HW. Age and outcome of superficial bladder cancer treated with Bacille Calmette–Guérin therapy. Urology 2007;70:65–9. [DOI] [PubMed] [Google Scholar]
- [10].Margel D, Alkhateeb SS, Finelli A, et al. Diminished efficacy of Bacille Calmette-Guérin among elderly patients with nonmuscle invasive bladder cancer. Urology 2011;78:848–54. [DOI] [PubMed] [Google Scholar]
- [11].Oddens JR, Sylvester RJ, Brausi MA, et al. The effect of age on the efficacy of maintenance Bacillus Calmette-Guérin relative to maintenance epirubicin in patients with stage Ta T1 urothelial bladder cancer: results from EORTC genito-urinary group study 30911. Eur Urol 2014;66:694–701. [DOI] [PubMed] [Google Scholar]
- [12].Cormio L, Sanguedolce F, Cormio A, et al. Human epidermal growth factor receptor 2 expression is more important than Bacillus Calmette Guerin treatment in predicting the outcome of T1G3 bladder cancer. Oncotarget 2017;8:25433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sanguedolce F, Cormio A, Massenio P, et al. Altered expression of HER-2 and the mismatch repair genes MLH1 and MSH2 predicts the outcome of T1 high-grade bladder cancer. J Cancer Res Clin Oncol 2018;144:637–44. [DOI] [PubMed] [Google Scholar]
- [14].Sanguedolce F, Brunelli M, D’amuri A, et al. Evolving concepts and use of immunohistochemical biomarkers in flat non-neoplastic urothelial lesions: WHO 2016 classification update with diagnostic algorithm. Biomarkers 2018;23:305–14. [DOI] [PubMed] [Google Scholar]
- [15].Sanguedolce F, Cormio A, Bufo P, et al. Molecular markers in bladder cancer: Novel research frontiers. Crit Rev Clin Lab Sci 2015;52:242–55. [DOI] [PubMed] [Google Scholar]
- [16].Cormio L, Tolve I, Annese P, et al. Altered p53 and pRb expression is predictive of response to BCG treatment in T1G3 bladder cancer. Anticancer Res 2009;29:4201–4. [PubMed] [Google Scholar]
- [17].Cormio L, Tolve I, Annese P, et al. Retinoblastoma protein expression predicts response to bacillus Calmette-Guerin immunotherapy in patients with T1G3 bladder cancer. Urol Oncol 2010;28:285–9. [DOI] [PubMed] [Google Scholar]
- [18].Cormio A, Sanguedolce F, Musicco C, et al. Mitochondrial dysfunctions in bladder cancer: exploring their role as disease markers and potential therapeutic targets. Crit Rev Oncol Hematol 2017;117:67–72. [DOI] [PubMed] [Google Scholar]
- [19].Sanguedolce F, Cormio A, Bufo P, et al. Predictive markers in bladder cancer: do we have molecular markers ready for clinical use? Crit Rev Clin Lab Sci 2014;51:291–304. [DOI] [PubMed] [Google Scholar]
- [20].Heiner JG, Terris MK. Effect of advanced age on the development of complications from intravesical bacillus Calmette-Guérin therapy. Urol Oncol 2008;26:137–40. [DOI] [PubMed] [Google Scholar]
- [21].Racioppi M, Di Ginafrancesco L, Ragonese M, et al. The challenges of Bacillus of Calmette-Guerin (BCG) therapy for high risk non muscle invasive bladder cancer treatment in older patients. J Geriatric Oncol 2018. [DOI] [PubMed] [Google Scholar]
- [22].Oddens JR, Sylvester RJ, Brausi MA, et al. Increasing age is not associated with toxicity leading to discontinuation of treatment in patients with urothelial non-muscle-invasive bladder cancer randomised to receive 3 years of maintenance bacille Calmette-Guérin: results from European Organisation for Research and Treatment of Cancer Genito-Urinary Group study 30911. BJU Int 2016;118:423–8. [DOI] [PubMed] [Google Scholar]


