Abstract
Women attending antenatal care (ANC) are a generally healthy, easy-access population, contributing valuable data for infectious disease surveillance at the community level. ANC-level malaria surveillance would provide a routine measure of the malaria burden in pregnancy, which countries lack, whilst potentially improving pregnancy outcomes. It could also offer contemporary information on temporal trends and the geographic distribution of malaria burden as well as intervention coverage in the population to guide resource allocation and to assess progress towards elimination. Here, we review the factors underlying the relationship between Plasmodium falciparum in pregnancy and in the community, and outline strengths and limitations of an ANC-based surveillance in sub-Saharan Africa, its potential role within wider malaria surveillance systems and subsequent programmatic applications.
Keywords: Malaria, pregnancy, antenatal care, surveillance
Surveilling malaria
The burden of malaria is highly heterogeneous at a range of spatial scales, driven by ecological and socio-economic factors that affect access to preventive interventions and treatment [1, 2]. As a result, the detection, characterization and monitoring of malaria through strategic use of surveillance (see Glossary) data is needed to inform optimal and equitable allocation of interventions tailored to local patterns of risk [3, 4].
Despite substantial improvements in diagnosis and reporting systems in many countries, estimating malaria trends directly from passive detection of clinical malaria cases at health facilities remains difficult due to challenges associated with availability of access to care and the estimation of denominator populations [5]. Hence, routine surveillance data are currently judged too unreliable by World Health Organization to estimate national-level malaria cases and deaths in most countries in Africa [6]. Instead, estimates of burden, alongside other indicators such as intervention uptake, are commonly based upon infection prevalence in children aged under 5, collected through nationally-representative household surveys. However, their expense means that these surveys are typically carried out every 2–3 years are lack power to estimate prevalence beyond the first administrative unit as well as to detect trends when transmission declines to low levels [7], limiting the extent to which resources can be allocated effectively in a timely and targeted manner. Moreover, current surveillance systems lack tools to monitor prevention efforts in pregnant women, a key risk group in which malaria infection can lead to anaemia, low birthweight and stillbirth, and contribute to neonatal mortality [8]. This is particularly the case in areas of high transmission, when negative outcomes will often occur in women who experience infection asymptomatically [9].
Surveillance strategies that target convenient sub-populations have been suggested as a cost-efficient approach to obtain more reliable information on malaria burden at a more operationally relevant spatial and temporal resolution [10]. Convenience sampling has been used successfully for infectious diseases such as schistosomiasis (school-based surveys [11]) or arbovirus (blood donor screening [12]). As a generally healthy population attending a routine health system contact, information from women at antenatal care (ANC) clinics have provided valuable information about infectious disease trends, such as HIV [13] and syphilis [14].
Integrating malaria surveillance at first ANC visit constitutes a potential easy-to-implement and cost-effective approach to provide a continuous measure of the malaria burden in pregnancy as well as to complement classical surveys for tracking malaria transmission in the community. In this opinion article, we consider epidemiological and immunological evidence that suggest that Plasmodium falciparum estimates obtained from pregnant women are representative of trends in the community (children under 5 or 2 to 10 years of age), point to factors that affect this relationship and discuss strengths, limitations as well as programmatic applications where ANC-based malaria surveillance could provide added value to existing surveillance systems in sub-Saharan Africa.
The relationship between malaria in pregnancy and in children
Studies conducted in the 50s in Sierra Leone showed that pregnant women exhibited the same prevalence of P. falciparum infection and corresponding seasonal changes as schoolchildren aged 5–7 years [15]. Similarly, trends of P. falciparum infection among pregnant Mozambican women studied between 2003 and 2012 mirrored trends in malaria-related outpatient visits [16]. A meta-analysis of studies conducted in Africa showed a strong correlation between P. falciparum prevalence in pregnant women, mostly from community-based surveys, and children from the same population [17]. Malaria prevalence among pregnant women at first ANC visit and infants nine to 12 months old attending routine health services for measles vaccination showed a good spatial and temporal correlation in a pilot study conducted in Tanzania [18]. A strong relationship has been estimated between clinical incidence of malaria in children under 5 years and ANC prevalence in the Democratic Republic of Congo between 2010 and 2016 [19]. Overall, these studies support the notion that measures of malaria obtained from pregnant women reflect malaria trends among children in the same community.
Prevalence of malaria infection as measured by slide microscopy (SM) is generally higher than in non-pregnant women within the same population [20]. Increased attraction of mosquitoes during pregnancy [21] has been implicated in this enhanced susceptibility, as well as physiological and immune adaptations required to accommodate the fetus [22]. However, differences in parasite prevalence and density by gravidity and gestational time highlight the key role of a malaria-specific immune responses [20], in particular those directed against antigenic variants presented by P. falciparum parasites that accumulate in the placenta [23]. Such placental sequestration is mediated by binding of parasite VAR2CSA [24], a 350 kDa multi-domain variant antigen expressed on the surface of infected erythrocytes, to chondroitin sulfate A (CSA) in the syncytiotrophoblast [25]. In conditions of high transmission, development of immunity against VAR2CSA after exposure to placental parasites is associated with a parity-dependent reduction in the prevalence and density of infection [9]. This acquisition of immunity likely contributes to the observation that survey prevalence in pregnant women, as measured by rapid diagnostic tests (RDTs) or SM, is similar to that in young children in areas of low transmission, whereas in areas of high transmission prevalence in pregnancy is substantially lower than in children, due primarily to low prevalence in multigravidae [17]. Further research is needed to understand the impact of coinfections and comorbidities that affect antimalarial immunity and thus the risk of malaria in pregnancy, such as HIV [26], helminths [27] and malnutrition [28], on the relationship of P. falciparum prevalence among pregnant women and children.
Low density parasitemia is likely to be common at the beginning of pregnancy due to infections acquired prior to conception [29–31]. However, particularly in primigravidae, these infections multiply to high densities after being selected for binding to CSA in the placenta, increasing the likelihood of detection using SM or RDTs. If infections observed during pregnancy mainly result from a boosting of those acquired before pregnancy [31], trends in prevalence among pregnant women may lag behind prevalence in children. Moreover, as malaria in pregnancy burden in high transmission settings is driven by asymptomatic prevalent infection, it is likely to lag substantially behind patterns of clinical disease in the general population. This is consistent with the findings from Democratic Republic of Congo where high clinical incidence rates in under 5 s were associated with an increased risk of a positive RDTs in pregnant women for the next 3 months [19] and in seasonal settings where women remain at high risk if infection at ANC throughout the dry season [29].
ANC-based testing as a routine surveillance resource
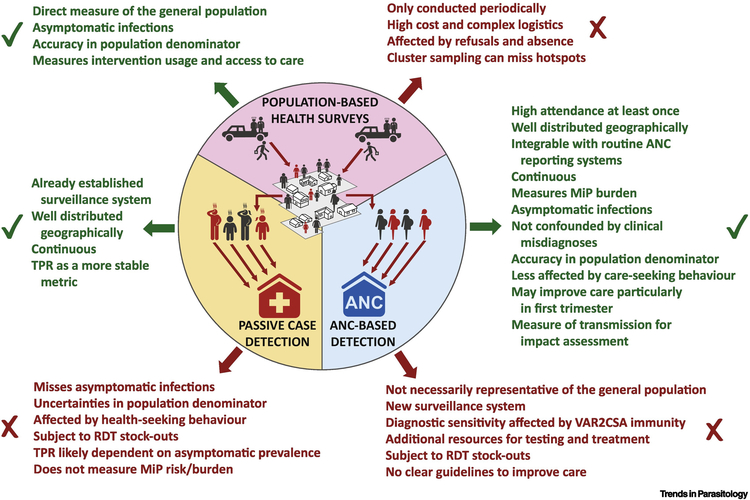
Although an easy-access and relatively low-cost data source, limited resources require that the benefits of implementing ANC-based surveillance (i.e., collecting the result of a malaria test and administering a questionnaire) are considered alongside approaches to improve existing passive case-reporting systems (Figure 1). The potential benefits of a continuous surveillance approach at ANC clinics to complement existing surveillance systems in Africa are manifold.
Figure 1. Advantages and disadvantages of using women at ANC clinics as sentinel populations for malaria surveillance over other surveillance methods.
The figure represents the pros and cons of P. falciparum surveillance at ANC clinics compared to main surveillance approaches operating in sub-Saharan Africa. The primary source to estimate malaria burden in Africa has been population-based health surveys (top), usually undertaken at the province level at widely spaced intervals due to operational challenges and high cost. Passive surveillance of malaria cases (left) through appropriate health management information systems (HMIS) offers more granular and contemporaneous data on a routine basis. Although HMIS are already stablished in most African countries and are a key focus of National Malaria Control Programs, the quality of data has often been poor with a general lack of timeliness, completeness and accuracy [61, 62]. Moreover, meaningful incidence rates require good estimates of the size of the health catchment population, which is unlikely to be available in many parts of sub-Saharan Africa [63]. As an alternative to incidence, test positivity rate (TPR) is likely to be affected by the rates of non-malarial febrile illness [37]. Thus, any estimates of burden derived from passive case systems are likely to be highly sensitive to health seeking behaviors, the decisions to test and the burden of asymptomatic infections. An ANC-based surveillance of malaria (right) would require to set up a new surveillance system and additional resources for testing and treatment. However, the numerical superiority and continuous nature of ANC data, compared to standard national surveys, would likely provide substantial potential applications while being less affected by variations in health seeking behaviors, as women do attend ANC clinics because they are pregnant rather than due to illness. Routine testing at first ANC visit would also reduce biases associated with decisions to test and provide information about asymptomatic infections. Moreover, as malaria surveillance at ANC clinics would measure prevalence, the estimates would be more stable to misspecification of population denominator, offering a valuable information to complement existing surveillance systems in Africa. Abbreviations: ANC, Antenatal care; MiP: Malaria in pregnancy; RDT: Rapid diagnostic test; TPR: Test positivity rate.
Monitoring malaria in pregnancy
Though empirically highly correlated, the relationship between P. falciparum prevalence in children and in pregnant women appears non-linear [17], likely due, at least in part, to the effects of acquired immunity to placental infection. Within settings with highly seasonal transmission, the high risk of malaria in pregnancy throughout dry seasons [29] supports previous modelling [32] in suggesting that declines in malaria burden in the wider population will not necessarily be directly proportional to those in pregnancy. This highlights the value of monitoring malaria in pregnancy as an independent, direct, measure of malaria burden. Capturing disparities in the prevalence in pregnant women and the general population within a ‘dashboard’ of routine malaria indicators could help policy-makers to make more informed decisions about resource allocation and equity, particularly given the low uptake of long-lasting insecticidal nets in women immediately prior to their first pregnancy [33]. When using ANC-based data for the wider population, these disparities become biases that would need to be accounted for, analogous to the well-known fertility biases inherent in using HIV prevalence in pregnancy for sentinel purposes [34]. In particular, adjusting for gravidity or restricting analysis to primigravidae [17] may improve the ability of ANC data to capture population-level prevalence trends.
Triangulation of malaria trends
ANC coverage and uptake is high, and improving, in sub-Saharan Africa (Box 1), with most women attending ANC clinics at least once during pregnancy, even in many hard-to-reach rural areas in Africa [35]. As the contact with the health facility is driven by pregnancy rather than illness, ANC data is probably less subject to biases associated with variations in healthcare seeking behavior than measures obtained through cases reported within the same health facility [36]. Testing generally healthy women at their first scheduled ANC visit, before they have received standard malaria prevention given at antenatal clinics, could provide a measure of asymptomatic prevalence. Routine testing within a scheduled ANC contact would reduce biases associated with decisions to test, especially as strategies and criteria for identifying suspected malaria cases can differ by setting [5]. Similarly, ANC surveillance would not be affected by rates of non-malarial febrile illness [37], which are likely to influence the malaria test positivity rate (TPR) in individuals with fever [38]. This would, however, ideally require an appropriate data entry and health management information system to distinguish between positive infections within women being tested routinely at a first scheduled visit from the testing of pregnant women with malaria symptoms during unscheduled visits or those later in pregnancy. ANC data could thus provide a means by which to benchmark trends within cases at the facility level, allowing triangulation with changes in prevalence between nationally representative health surveys, and offer complementary information to elucidate real underlying changes in malaria burden or transmission. Finally, women at ANC clinics could also be conveniently surveyed about the use of malaria preventive measures, potentially allowing a real-time assessment of intervention coverage and progress in program implementation, in contrast to information collected from more sporadic national surveys (Figure 2). As a result, ANC-based surveillance could substantially improve the returns on investments countries have made in upgrading case reporting systems and wider health system strengthening. However, malaria surveillance at ANC clinics would still require documentation of the effects of antenatal seeking rates and practices, including the impact that private ANC clinics and fee for service models can have on the representativeness and generalizability of the data (Box 2), as part of the validation process.
Box 1: Use of ANC services in sub-Saharan Africa.
Antenatal care (ANC) aims to prevent or ensure early treatment of pregnancy complications through systematic assessments, women’s education, screening for fetal development and early detection of mother and baby abnormalities [55]. ANC is universally accepted to be the most important determinant of pregnancy outcomes, is strongly associated with a reduction in maternal deaths [56] and represents an important entry point for different programs and provision of integrated care, including: a) Identification and management of obstetric complications, reproductive tract infections, HIV and anemia; b) Vaccination with recommended vaccines; c) Malaria prevention (insecticide-treated mosquito nets and IPTp with SP [57], except for women at first trimester given safety contraindications of SP) in all areas with moderate to high malaria transmission in Africa; and d) Advice on nutrition including micronutrient supplements such as iron and folic acid.
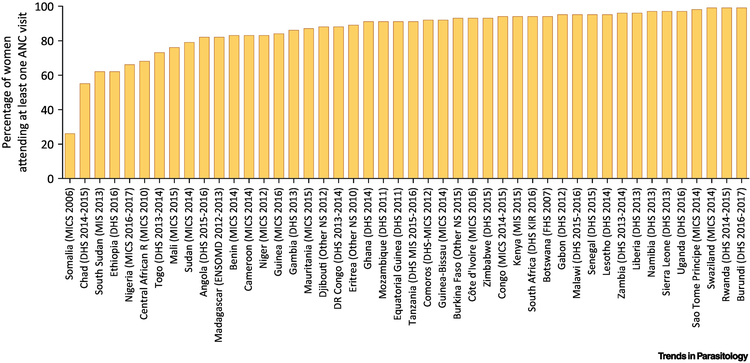
WHO currently recommends a “standard” model of attaining at least eight ANC visits [58]. However, inequity in ANC persists [59]. Globally, 86% of pregnant women worldwide access antenatal care with skilled health personnel at least oncei, being more than 95% in industrialized countries, 78% in sub-Saharan Africa [35] (Figure I) and 69% in South Asia. However, only 62% globally receive at least four antenatal care visits (52% in sub-Saharan Africa)i. Moreover, in Africa, 80% of women in the richest quintile have access to three or more ANC visits, while only 48% of the poorest women have the same level of access. A similar disparity exists between urban and rural womenii. Innovative strategies targeting financial and cultural factors are needed to increase access and uptake of ANC.
Figure I.
ANC attendance in sub-Saharan Africa (data obtained from iii)
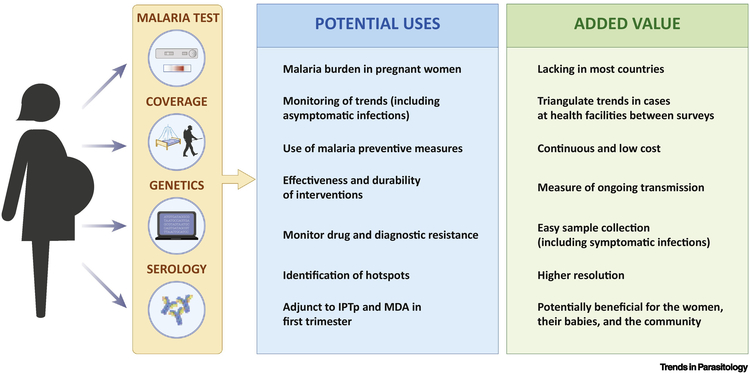
Figure 2. Potential uses and added value of an ANC-based surveillance for malaria.
At first antenatal contact, data on malaria infection can be collected in a continuous way as part of routine services without requiring any additional sampling, together with screening for HIV, syphilis, and anaemia. Malaria screening at ANC would provide a routine measure of the malaria burden in pregnancy, which countries lack, whilst potentially improving pregnancy outcomes. As ANC clinics are well distributed and most African women attend them at least once, they could potentially increase resolution and precision of estimates generated through national surveys. Continuous (year-round) surveillance at ANC clinics may provide contemporary information on temporal trends, the geographic distribution of malaria burden, signals of sustained decreases in transmission due to an intervention (e.g, seasonal malaria chemoprevention) and early warning signals to rises in transmission. The recording of information about the use of malaria preventive measures could potentially allow a real-time assessment of intervention coverage in the population to guide resource allocation and to assess progress towards elimination. Antibodies against VAR2CSA, which are developed after exposures to placental parasites, may provide an adjunct to detect recent infections or document historical changes in transmission. Finally, women attending ANC may also constitute a convenient sampling population for the assessment of the parasite genetic make-up (i.e., complexity of infection), drug resistance gene flow between populations and adaptations developed by the parasite to control strategies, such as antimalarial resistance and deletions of antigens targeted by rapid diagnostic test that can compromise diagnosis, treatment and prevention.
Box 2: Representativeness and generalizability.
The extent to which pregnant women attending antenatal clinics are representative of all pregnant women in a country is affected by non-attendance at antenatal clinics, attendance at private clinics, and the location of surveillance clinics.
ANC attendance: The use of ANC clinics may be linked to demographic characteristics (e.g. place of residence, educational level, socioeconomic status or parity) that could also be related to malaria risk. Women who do not attend antenatal clinics are often more rural, less literate, and older than women who utilize antenatal clinics. This source of selection bias is likely to be small in malaria-endemic Africa where 78% women attend an antenatal clinic at least once [35]iv. In countries where this is not the case, hybrid prevalence estimators that combine data from relatively small random community surveys with the convenience sample obtained from ANC clinics could provide more accurate information, as has been done for HIV [60].
Rural pregnant women: Including ANC serving pregnant women in rural areas, which are often remote, smaller and with low client volume, can be challenging. However, underrepresentation of rural ANC attendees may bias estimates downwards, as malaria prevalence is higher in these women.
Attendance at private ANC clinics: In countries where the number of pregnant women accessing private ANC is large or growing, underrepresentation of private clinics in surveillance could introduce bias if the populations of pregnant women attending private and public ANC differ with regards to malaria exposure.
In general, representativeness of ANC surveys may critically depend on the level and equity of antenatal care as well as on health-seeking behaviors and attitudes. Other potential biases to take into account include participation bias (if choice in who gets tested is allowed), selection bias (if pregnant women whose routine data are collected for surveillance differ in an important way from pregnant women whose data are not collected), information bias (if surveillance design, methods or structure, quality or availability of data collected changes during timer) and measurement bias (if there are systematic errors in the measurement or classification of surveillance parameters or variables, such as malaria-testing results).
Increasing resolution
Up-to-date estimates of sub-national burden are critical when it comes to the development of national strategic plans. Data from ANC clinics, which are well-distributed geographically, could provide guidance for tailoring sampling schemes to underlying patterns of risk, especially when malaria prevalence is low and powering surveys to detect trends becomes impractical even at the level of the first administrative unit. The resolution achieved by an ANC-surveillance approach would depend on the distribution and sample sizes achievable at ANC clinics at a particular location and time-point, which are naturally constrained by the number of women searching for ANC. Assuming 3–5% annual pregnancies in most African populations, an 80% attendance rate for at least one ANC visit and a 20–30% proportion of first-time pregnancies, between 0.8 and 1.4 million data points (0.2 to 0.4 million of these from primigravid women) would be expected annually from a country with 35 million inhabitants. For comparison, as of July 2018, the Malaria Atlas Project v, which attempts to archive all P. falciparum and P. vivax malaria prevalence survey data collected globally since 1975, contains data from 5,188,396 people examined. As a result, the likely numerical superiority of ANC-based surveillance compared to standard national surveys will not be affected by reductions infertility rates reported in Africa vi, especially given that fertility rates are usually higher in rural areas where malaria is most concentrated and that the per-capita rate of first pregnancies is likely to remain relatively constant. Thus, testing all ANC attendees within a first administrative unit in a given year, the resolution at which population-based surveys are typically powered, could provide sample size orders of magnitude above than those financially feasible within a survey. Those numbers would allow much more precise estimation of trends and prevalence at a more operationally relevant resolution, such as the second administrative level or beyond.
Adjunct for elimination
As transmission declines, the geographical distribution of P. falciparum infections becomes more heterogeneous [2]. This increases the potential error associated with population-based survey approaches due to inadequate sampling of infection hotspots within randomly selected survey clusters. In settings in which malaria is being eliminated, ANC-based malaria surveillance has the potential to contribute for the identification of foci of continuing transmission which are not captured by case-based surveillance, and to help disentangle situations where transmission is self-sustaining from those driven by imported cases who seek care. This could allow a better targeting of residual pockets of transmission using interventions such as indoor residual spraying or focal drug administration.
Measuring impact
The incremental benefit of having an additional measure of population prevalence such as ANC-based surveillance in addition to case-reporting systems will also depend strongly upon the combinations of interventions within a setting and the extent to which they are likely to affect the relationship between malaria transmission and malaria burden. For example, settings implementing seasonal malaria chemoprevention in children, where TPR is likely to be a particularly unreliable indicator of endemicity, are likely to benefit from a measure of ongoing transmission when attempting to assess the effectiveness of this intervention. Similarly, in settings implementing mass drug administration, an intervention in which pregnant women are deliberately excluded, measuring trends in asymptomatic infection would provide an indication of the impact upon transmission over-and-above the direct impact of chemoprophylaxis.
Other applications from ANC surveillance using alternative assays
As a continuous, well-distributed and easy-access group, women attending ANC clinics provide an attractive sampling population for measures of exposure to malaria beyond simply presence or absence of parasite infection (Figure 2). The molecular analysis of P. falciparum isolates collected from pregnant women may provide a means to characterize genetic signatures of malaria transmission intensity (complexity of infection), gene flow and parasite adaptations to avoid clearance (antimalarial resistance) and detection (deletions of antigens targeted by rapid diagnostic test) [39, 40]. Future studies should test if the specific selection of parasites in the placenta leads to a difference in the prevalence of such parasite markers in pregnant women and children (Outstanding questions).
As pregnant women develop antibodies against VAR2CSA after single or very limited exposures to placental parasites [41], appropriately chosen antibodies against immunogenic non-polymorphic epitopes could provide information about a woman’s history of exposure during a very specific window of exposure (i.e., a pregnancy) [42]. Because primigravidae have not previously been exposed to placental type parasites, seropositivity for VAR2CSA would indicate an infection acquired during the period of pregnancy elapsed until sampling. As different VAR2CSA domains elicit IgG responses with varying magnitudes and dynamics [43, 44], VAR2CSA sero-surveillance could be tuned to different uses based on the half-life of the antibodies targeted. The use of a point of care serological test to detect short half-lived antibodies against VAR2CSA in pregnant women or among recently pregnant women attending health facilities for infant immunization would allow to quantify recent infections, while detection of IgG responses of longer duration may be useful to document historical changes in transmission by sampling multigravidae or women of child-bearing age. Understanding the value of that data over parasite prevalence for a more sensitive estimation of malaria transmission, for the detection of hotspots or as a cost-efficient adjunct approach to document freedom from infection [45] needs to be determined.
Clinical considerations
Ensuring optimal care for women who provide the data at ANC clinics would be of paramount importance and, by participating in testing, women should receive at least the standard of care they would receive in the absence of the test. From the second trimester onwards in African pregnant women, this would be intermittent preventive treatment during pregnancy (IPTp) with Sulfadoxine-Pyrimethamine (SP). RDT-negative women may still harbor low-density infection and will gain benefit from receiving SP, both from having any such parasites cleared and from the prophylaxis period against subsequent new infection the drug confers [46]. As a result, these women should not be left unprotected and should continue to receive IPTp with a drug at least as efficacious as SP. For test-positive women, the choice of treatment drug needs to be considered carefully both in terms of the likelihood the drug will clear infection and the period of prophylaxis it subsequently provides relative to SP, which itself will depend upon the degree of parasite resistance to SP [33]. Particular care will be necessary in West Africa, where SP is likely to have a longer prophylactic profile than some artemisinin combination therapies used for first-line treatment such as artemether-lumefantrine [47]. Outside of Africa, where IPTp is generally not provided, routine testing may have substantial benefits for women with asymptomatic infection. Routine screening for infection at first ANC visit for this purpose is already national policy in Indonesia [48].
ANC surveillance may also contribute to improve the health of the approximately 24% pregnant women in sub-Saharan region who begin ANC in the first trimester [49], a number that might increase during next years given improved utilization and quality of ANC [50]. Malaria infections in the first trimester are highly prevalent [9, 30, 51] and associated with maternal anemia, miscarriages and low birthweight [51, 52]. As IPTp with SP cannot be given to women in the first trimester [53], ANC-surveillance could provide benefit to the tested women by identifying and treating those with asymptomatic but detectable parasitemias who are excluded from IPTp due to drug safety concerns.
Concluding Remarks
There is an urgent need to optimize the impact of malaria control and elimination strategies in countries through strategic use of easily accessible information. The integration of malaria surveillance at ANC clinics with other surveillance systems could be beneficial not only to better characterize malaria infection and prevention in pregnant women, and subsequent impact on maternal and neonatal health, but also as a source of actionable information for malaria control and elimination within the wider population. It could also lead to better care, and more informed decision-making around malaria prevention, for the woman and her baby. Continuous routine screening of pregnant women at ANC clinics is likely to cause relatively little disruption to clinic flow, as pregnant women already have blood drawn for routine ANC screening. Increases in health staff workload associated with additional testing in an already strained environment may be resolved with experience and proficiency with the testing procedures [54]. However, other bottlenecks to scale-up an ANC-based surveillance, such as test stock-outs and the need of funding commitment, need be considered. More studies are required to better characterize the relationship between malaria infection in pregnant women and in less than 5 or 2–10 year olds children, as well as the optimal approach and minimum data required to infer population-level trends from routine ANC-based testing (see Outstanding Questions). However, antenatal malaria surveillance has a high potential to complement household surveys as a source of much higher spatial and temporal resolution prevalence data to better monitor progress towards control targets and to tailor interventions according to local levels of transmission.
OUTSTANDING QUESTIONS.
What is the optimal approach to estimate the clinical burden of malaria in the general population from malaria prevalence in pregnant women? How do gravidity, gestational age and comorbidities (malnutrition, co-infections) affect this relationship?
What are the social and behavioral determinants of ANC use that can affect the representativeness and generalizability of malaria metrics obtained at antenatal clinics? Does the care seeking behavior of pregnant women and the coverage of ANC clinics lead to less biased estimates of malaria burden than those obtained from sick children at outpatient departments?
Which is the optimal ANC surveillance design to provide representative estimates of malaria in the community? What sample-size is needed to maximize precision for a given cost? How to effectively integrate data from ANC surveillance, community surveys and clinical cases to guide decision-making for malaria control and elimination?
How similar is the information on intervention coverage obtained from pregnant women and from population-based surveys?
Does pregnancy-specific serology provide added value to generate estimates of malaria and to take action?
Are parasite populations within pregnant women representative of the general population and can they be used for genetic surveillance (e.g. antimalarial resistance makers, gene deletions, complexity of infection, genetic polymorphisms of vaccine candidates)?
What threshold of detection is needed to produce precise malaria estimates? Which is the best diagnostic tool to produce actionable surveillance data and benefit pregnant women?
What are the healthcare workers’ and pregnant women’s perceptions on the appropriateness, acceptability and feasibility of an ANC-based surveillance of malaria? Which is the cost associated with the large-scale implementation of such a surveillance approach?
Highlights.
Patterns of malaria prevalence in pregnant women show strong correlation with those observed in children, although the relationship is affected by variables such as the intensity of malaria transmission, parity and immunosuppressive conditions.
Malaria screening at antenatal care (ANC) would provide a routine measure of the malaria burden in pregnancy, missing in most countries, whilst potentially improving pregnancy outcomes.
ANC may offer an effective basis to provide actionable information for analysis of malaria trends, stratification and planning of resource allocation, as well as an adjunct for approaches aiming to investigate foci of transmission and measure progress towards elimination.
ANC-based prevalence could benchmark case reporting trends at health-facilities and triangulate with changes in prevalence between surveys, allowing routine data to better inform global burden estimation.
Acknowledgements
This work was supported by the National Institute of Health (1R01AI123050–01: Pregnant women as a sentinel group for malaria surveillance in an era of changing malaria transmission) and the Departament d’Universitats i Recerca de la Generalitat de Catalunya (AGAUR; grant 2017 SGR 664). ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya (http://cerca.cat/en/suma/). CISM is supported by the Government of Mozambique and the Spanish Agency for International Development (AECID). The funders had no role in the preparation of this manuscript. We would like to thank Caterina Guinovart and Beatriz Gálatas for helpful inputs at earlier stages of the work.
GLOSSARY
- Antenatal care (ANC)
A type of preventive healthcare which aims to provide regular check-ups that allow doctors or midwives to treat and prevent potential health problems throughout the course of the pregnancy and to promote healthy lifestyles that benefit both mother and child.
- Asymptomatic malaria
Presence of parasites in the blood not accompanied by symptoms of malaria.
- Convenient population
Individuals easily accessible because they are present in a central locale.
- Coverage
A general term referring to the fraction of the population of a specific area that receives a particular intervention.
- Health seeking behaviour
Any action undertaken by individuals who perceive themselves to have a health problem or to be ill for the purpose of finding an appropriate remedy.
- Hotspots
Areas where malaria transmission intensity exceeds the average level.
- Intermittent preventive treatment in pregnancy (IPTp)
A full therapeutic course of antimalarial medicine given to pregnant women at routine antenatal visits, regardless of whether the woman is infected with malaria, currently recommended by WHO in all areas with moderate to high malaria transmission in Africa.
- Malaria test positivity rate
Proportion of diagnostic tests that are positive for malaria. It is an alternate indicator of malaria morbidity to overcome the challenges of interpreting routine surveillance data in settings where testing rates are variable or denominator populations are hard to define.
- Mass drug administration
Administration of antimalarial treatment to all age groups of a defined population or every person living in a defined geographical area (except those for whom the medicine is contraindicated) at approximately the same time and often at repeated intervals.
- Passive-detection systems
Detection of malaria cases among patients who, on their own initiative, visit health services for diagnosis and treatment, usually for a febrile illness.
- Population-based survey
Survey conducted in a representative sample of selected age groups to estimate the prevalence of malaria and coverage of interventions. Current standards for such surveys are malaria indicator surveys and related demographic and health surveys or multiple indicator cluster surveys.
- Seasonal malaria chemoprevention
Intermittent administration of full treatment courses of an antimalarial medicine to children during the malaria season to prevent malarial illness. This intervention is recommended only for areas with highly seasonal malaria, where transmission occurs during a few months of the year.
- Surveillance
Continuous, systematic collection, analysis and interpretation of disease-specific data and use in planning, implementing and evaluating public health practice. Surveillance can be done at different levels of the health care system (e.g. health facilities, the community), with different detection systems (e.g. case-based: active or passive) and sampling strategies (e.g. sentinel sites, surveys).
- VAR2CSA
A variant antigen belonging to the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family that mediates parasite sequestration in the placental though its adhesion to chondroitin sulfate A (CSA).
RESOURCES
UNICEF Unicef Global Databases: https://data.unicef.org/topic/maternal-health/antenatal-care/.
UNICEF (2015) Progress for Children Beyond Averages: Learning from the MDGs: ≤https://data.unicef.org/resources/progress-for-children-report≥,. New York.
UNICEF (2017) Monitoring the Situation of Children and Women: https://data.unicef.org/topic/maternal-health/antenatal-care/#.
UNICEF global databases 2017 based on MICS and DHS: https://data.unicef.org/topic/maternal-health/antenatal-care/.
The Malaria Atlas Project: https://map.ox.ac.uk/
United Nations, Department of Economic and Social Affairs, Population Division, Population Division World Population Prospects 2019: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bhatt S et al. (2015) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526 (7572), 207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousema T et al. (2012) Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med 9 (1), e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gething PW et al. (2014) Declining malaria in Africa: improving the measurement of progress. Malar J 13, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (2015) Global Technical Strategy for Malaria 2016–2030 Geneva. [Google Scholar]
- 5.World Health Organization (2018) Malaria surveillance, monitoring & evaluation: a reference manual Geneva. [Google Scholar]
- 6.World Health Organization (2018) World Malaria Report 2018 Geneva. [Google Scholar]
- 7.Hay SI et al. (2008) Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis 8 (6), 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai M et al. (2018) Prevention of malaria in pregnancy. Lancet Infect Dis 18 (4), e119–e132. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson SJ et al. (2018) Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 18 (4), e107–e118. [DOI] [PubMed] [Google Scholar]
- 10.Oduro AR et al. (2011) Health centre surveys as a potential tool for monitoring malaria epidemiology by area and over time. PLoS One 6 (11), e26305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortu G et al. (2017) Countrywide Reassessment of Schistosoma mansoni Infection in Burundi Using a Urine-Circulating Cathodic Antigen Rapid Test: Informing the National Control Program. Am J Trop Med Hyg 96 (3), 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chevalier MS et al. (2017) Use of Blood Donor Screening Data to Estimate Zika Virus Incidence, Puerto Rico, April-August 2016. Emerg Infect Dis 23 (5), 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwesigabo G et al. (2000) Monitoring of HIV-1 infection prevalence and trends in the general population using pregnant women as a sentinel population: 9 years experience from the Kagera region of Tanzania. J Acquir Immune Defic Syndr 23 (5), 410–7. [DOI] [PubMed] [Google Scholar]
- 14.Korenromp EL et al. (2018) Syphilis prevalence trends in adult women in 132 countries - estimations using the Spectrum Sexually Transmitted Infections model. Sci Rep 8 (1), 11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walton GA (1949) On the control of malaria in Freetown, Sierra Leone; control methods and the effects upon the transmission of Plasmodium falciparum resulting from the reduced abundance of Anopheles gambiae. Ann Trop Med Parasitol 43 (2), 117–39. [DOI] [PubMed] [Google Scholar]
- 16.Mayor A et al. (2015) Changing Trends in P. falciparum Burden, Immunity, and Disease in Pregnancy. N Engl J Med 373 (17), 1607–17. [DOI] [PubMed] [Google Scholar]
- 17.van Eijk AM et al. (2015) Prevalence of malaria infection in pregnant women compared with children for tracking malaria transmission in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 3 (10), e617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willilo RA et al. (2016) Pregnant women and infants as sentinel populations to monitor prevalence of malaria: results of pilot study in Lake Zone of Tanzania. Malar J 15 (1), 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellewell J et al. (2018) Using ante-natal clinic prevalence data to monitor temporal changes in malaria incidence in a humanitarian setting in the Democratic Republic of Congo. Malar J 17 (1), 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brabin BJ (1983) An analysis of malaria in pregnancy in Africa. Bull World Health Organ 61 (6), 1005–16. [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsay S et al. (2000) Effect of pregnancy on exposure to malaria mosquitoes. Lancet 355 (9219), 1972. [DOI] [PubMed] [Google Scholar]
- 22.Kourtis AP et al. (2014) Pregnancy and infection. N Engl J Med 371 (11), 1077. [DOI] [PubMed] [Google Scholar]
- 23.Brabin BJ et al. (2004) The sick placenta-the role of malaria. Placenta 25 (5), 359–78. [DOI] [PubMed] [Google Scholar]
- 24.Salanti A et al. (2003) Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol 49 (1), 179–91. [DOI] [PubMed] [Google Scholar]
- 25.Fried M and Duffy PE (1996) Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272 (5267), 1502–4. [DOI] [PubMed] [Google Scholar]
- 26.Mayor A et al. (2013) Improved pregnancy outcomes in women exposed to malaria with high antibody levels against Plasmodium falciparum. J Infect Dis 207 (11), 1664–74. [DOI] [PubMed] [Google Scholar]
- 27.Salgame P et al. (2013) Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol 14 (11), 1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaible UE and Kaufmann SH (2007) Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 4 (5), e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry I et al. (2018) Seasonal Dynamics of Malaria in Pregnancy in West Africa: Evidence for Carriage of Infections Acquired Before Pregnancy Until First Contact with Antenatal Care. Am J Trop Med Hyg 98 (2), 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker PG et al. (2013) A model of parity-dependent immunity to placental malaria. Nat Commun 4, 1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuikue Ndam N et al. (2018) Persistent Plasmodium falciparum Infection in Women With an Intent to Become Pregnant as a Risk Factor for Pregnancy-associated Malaria. Clin Infect Dis 67 (12), 1890–1896. [DOI] [PubMed] [Google Scholar]
- 32.Walker PG et al. (2014) Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health 2 (8), e460–7. [DOI] [PubMed] [Google Scholar]
- 33.Walker PG et al. (2017) Estimated impact on birth weight of scaling up intermittent preventive treatment of malaria in pregnancy given sulphadoxine-pyrimethamine resistance in Africa: A mathematical model. PLoS Med 14 (2), e1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregson S et al. (2015) Do HIV prevalence trends in antenatal clinic surveillance represent trends in the general population in the antiretroviral therapy era? The case of Manicaland, East Zimbabwe. AIDS 29 (14), 1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Eijk AM et al. (2013) Coverage of intermittent preventive treatment and insecticide-treated nets for the control of malaria during pregnancy in sub-Saharan Africa: a synthesis and meta-analysis of national survey data, 2009–11. Lancet Infect Dis 13 (12), 1029–42. [DOI] [PubMed] [Google Scholar]
- 36.Oduro AR et al. (2016) Monitoring malaria using health facility based surveys: challenges and limitations. BMC Public Health 16, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalrymple U et al. (2017) Quantifying the contribution of Plasmodium falciparum malaria to febrile illness amongst African children. Elife 6, e29198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francis D et al. (2012) Health facility-based malaria surveillance: the effects of age, area of residence and diagnostics on test positivity rates. Malar J 11, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniels RF et al. (2015) Modeling malaria genomics reveals transmission decline and rebound in Senegal. Proc Natl Acad Sci U S A 112 (22), 7067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Q et al. (2014) Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J 13, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayor A et al. (2011) Parity and placental infection affect antibody responses against Plasmodium falciparum during pregnancy. Infect Immun 79 (4), 1654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fonseca AM et al. (2019) Pregnancy-specific serology reveals temporal and spatial P. falciparum transmission patterns. Emerging Infectious Diseases In press. [DOI] [PMC free article] [PubMed]
- 43.Andersen P et al. (2008) Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog 4 (2), e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fowkes FJ et al. (2012) New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis 206 (10), 1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stresman G et al. (2017) Freedom from Infection: Confirming Interruption of Malaria Transmission. Trends Parasitol 33 (5), 345–352. [DOI] [PubMed] [Google Scholar]
- 46.Madanitsa M et al. (2016) Scheduled Intermittent Screening with Rapid Diagnostic Tests and Treatment with Dihydroartemisinin-Piperaquine versus Intermittent Preventive Therapy with Sulfadoxine-Pyrimethamine for Malaria in Pregnancy in Malawi: An Open-Label Randomized Controlled Trial. PLoS Med 13 (9), e1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okell LC et al. (2014) Contrasting benefits of different artemisinin combination therapies as first-line malaria treatments using model-based cost-effectiveness analysis. Nat Commun 5, 5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill J et al. (2018) Evaluation of the national policy of single screening and treatment for the prevention of malaria in pregnancy in two districts in Eastern Indonesia: health provider perceptions. Malar J 17 (1), 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. (2003) Antenatal care in developing countries: promises, achievements and missed opportunities: an analysis of trends, levels and differentials, 1990–2001
- 50.World Health Organization (2016) WHO recommendations on antenatal care for a positive pregnancy experience Geneva. [PubMed] [Google Scholar]
- 51.Accrombessi M et al. (2018) Effects of Malaria in the First Trimester of Pregnancy on Poor Maternal and Birth Outcomes in Benin. Clin Infect Dis [DOI] [PubMed]
- 52.Huynh BT et al. (2015) Burden of malaria in early pregnancy: a neglected problem? Clin Infect Dis 60 (4), 598–604. [DOI] [PubMed] [Google Scholar]
- 53.Goncalves BP et al. (2017) Pregnant Women: An Overlooked Asset to Plasmodium falciparum Malaria Elimination Campaigns? Trends Parasitol 33 (7), 510–518. [DOI] [PubMed] [Google Scholar]
- 54.Young N et al. (2019) Integrated point-of-care testing (POCT) for HIV, syphilis, malaria and anaemia at antenatal facilities in western Kenya: a qualitative study exploring end-users’ perspectives of appropriateness, acceptability and feasibility. BMC Health Serv Res 19 (1), 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chopra M et al. (2009) Saving the lives of South Africa’s mothers, babies, and children: can the health system deliver? Lancet 374 (9692), 835–46. [DOI] [PubMed] [Google Scholar]
- 56.Dowswell T et al. (2015) Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev (7), CD000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization (2004) A strategic framework for malaria prevention and control during pregnancy in the African region Brazzaville: World Health Organization Regional Office for Africa. [Google Scholar]
- 58.World Health Organization (2018) WHO recommendation on group antenatal care The WHO Reproductive Health Library; Geneva. [Google Scholar]
- 59.The Partnership for Maternal, Newborn and Child Health (2006) Opportunities for Africa’s newborns: Practical data, policy and programmatic support for newborn care in Africa. WHO on behalf of The Partnership for Maternal Newborn and Child Health
- 60.Hedt BL and Pagano M (2011) Health indicators: eliminating bias from convenience sampling estimators. Stat Med 30 (5), 560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maokola W et al. (2011) Enhancing the routine health information system in rural southern Tanzania: successes, challenges and lessons learned. Trop Med Int Health 16 (6), 721–30. [DOI] [PubMed] [Google Scholar]
- 62.Mavimbe JC et al. (2005) Assessing immunization data quality from routine reports in Mozambique. BMC Public Health 5, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amouzou A et al. (2013) Monitoring child survival in ‘real time’ using routine health facility records: results from Malawi. Trop Med Int Health 18 (10), 1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]