Abstract
We aimed to assess serial 18F-FDG PET/CT imaging according to morphological (RECIST1.1, iRECIST) and functional (PERCIST, PECRIT) criteria to predict clinical response to therapy in patients with advanced melanoma receiving immune checkpoint blocking agents.
Retrospective data collection and analysis was done for 37 patients with unresectable metastatic cutaneous melanoma eligible for immunotherapy (cycles: 4 for ipilimumab and pembrolizumab/ 6 for nivolumab).18F-FDG PET/CT imaging was performed prior to (18F-FDG PET/CT 0) and 14 weeks after ICI onset (18F-FDG PET/CT 1). Some cases during the follow-up required imaging (18F-FDG PET/CT 2). Assessment of patient response to treatment was done according to RECIST1.1, iRECIST, PERCIST and PECRIT criteria.
Among 37 assessed patients, 27 had 1 line of ICI, 8 had 2 lines of ICI and 2 patients had 3 lines of ICI: total of 49 PET/CTs. Mean time between initiation of ICI and 18F-FDG PET/CT (1 or 2) were respectively 13.82 ± 4.32 and 24.73 ± 9.53 weeks. Time between 18F-FDG PET/CT 1 and 18F-FDG PET/CT 2 was at mean +/− SD: 11.19w ± 5.59. Median PFS was 29.62 months (range 22.52–36.71) (P = .001: RECIST 1.1), (P < .0001: iRECIST), (P = .000: PERCIST), (P = .072: PECRIT). Median OS was 36.62 months (30.46–42.78) (P = .005: RECIST 1.1), (P < .0001: iRECIST), (P = .001: PERCIST), (P = .082 PECRIT).
18F-FDG PET/CT could detect eventual ICI-response in patients with metastatic melanoma undergoing ICI using iRECIST and PERCIST criteria
Keywords: criteria, FDG PET/CT, Immune checkpoint inhibitor, melanoma, response assessment
1. Introduction
The incidence of malignant melanoma has increased over the last decades [1] with a poor prognosis for patients with advanced tumor.[2] Immunotherapy, like immune checkpoint inhibitors (ICI) blocking CTLA-4 (e.g., ipilimumab), PD-1 (e.g., nivolumab, pembrolizumab) have demonstrated objective tumor regressions in patients with advanced melanoma and other types of cancer as non-small cell lung cancer (NSCLC) using the activation of the immune system to generate an anti-tumor response.[3–6] Computed Tomography (CT) assessment of some cancers under (immunotherapy) treatment shows baseline enlarged tumors that seem to fall back over time. Enlarged tumor size could be due to the infiltration and proliferation of lymphocytes and other immune cells. Other tumors remain stable in size for a prolonged time, even after therapy has been stopped.[7] Anatomic size measurements do not always appropriately capture a positive tumor response, particularly when the therapeutic agent under investigation stabilizes disease rather than causes tumor shrinkage, or when the study is performed in certain cancer types. Thus traditional RECIST1.1 morphological criteria may not be reliable to characterize clinical outcomes in cancer patients treated with immune-based anti-neoplastic drugs. Other treatment response criteria such as immune-related response criteria (irRC)[8] and iRECIST[9] are increasingly being used. Several studies have investigated the role of 18F-FluoroDeoxyGlucose Positron Emission Tomography/Computed Tomography (18F-FDG PET/CT) imaging in early detection of response to immunotherapy using characterization criteria such as PERCIST 1.0 and PECRIT.[10–12] Many studies have suggested that functional findings obtained from 18F-FDG PET/CT scans could be used ancillary to anatomic findings obtained by conventional spiral CT and Magnetic Resonance Imaging (MRI).[13] In the footsteps of the literature, we retrospectively analyzed serial 18F-FDG PET/CT imaging according to morphological (RECIST 1.1, iRECIST) and functional (PERCIST 1.0, PECRIT) criteria to predict clinical response to therapy (i.e., Progression Free Survival (PFS) and Overall Survival (OS)) in patients with advanced melanoma receiving immune checkpoint blocking agents.
2. Materials and methods
2.1. Patients
Thirty seven consecutive patients with unresectable metastatic cutaneous melanoma were seen at oncology consultation between November 2010 and June 2017 (23 men, 14 women). They were scheduled for ICI blocking CTLA-4 (e.g., ipilimumab) or PD-1 (e.g.,nivolumab, pembrolizumab) treatment.
Patients with uveal or mucosal melanoma or with only brain metastasis were excluded from analysis due to the known limitation of 18F-FDG PET/CT in detecting these lesions. Of the 37 patients, 13 had already been pretreated for metastatic melanoma, and 24 received no treatment while in stage IV. The pretreated patients received therapies containing one or more of the following: dacarbazine or formustine or BRAF inhibitor (BRAFi) or combination of BRAF inhibitor and MEK inhibitor (BRAFi+MEKi). The characteristics of the patients investigated are presented in Table 1.
Table 1.
Charac.
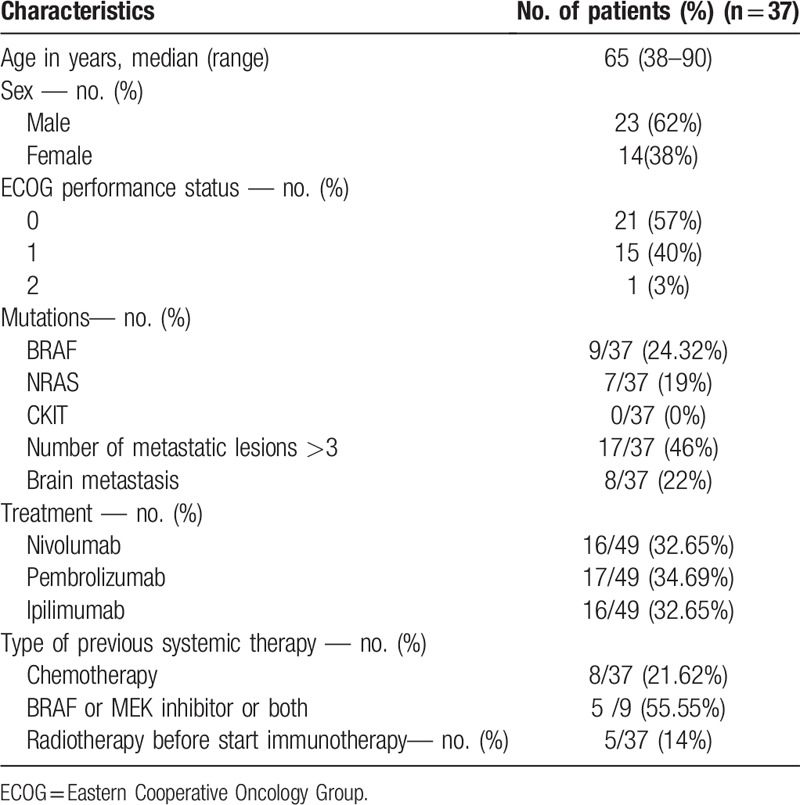
Ipilimumab was administered intravenously at a dose of 3 mg/kg every 3 weeks for a total of four doses. Nivolumab was administered intravenously at a dose of 3 mg/kg every 2 weeks and pembrolizumab was administered intravenously at a dose of 2 mg/kg every 3 weeks; anti-PD-1 was continued until progression or serious toxicity. Among 37 patients assessed by 18F-FDG PET/CT, 27 patients had 1 line of ICI, 8 patients had 2 lines of ICI and 2 patients had 3 lines of ICI thus 49 PET/CTs were performed.
Patients gave written informed consent to participate in the study and to have their medical records released. Retrospective collection and analysis of medical data was done from June 2017 to January 2018.
The study was approved by the local Ethics Committee of our institution and was registered on clinicaltrial.gov (ClinicalTrials.gov number NCT 03741231).
2.2. Data acquisition
18F-FDG PET/CT scans were performed on a Biograph mCT PET/CT 64 scanner system (Siemens, Erlangen, Germany) at baseline, and approximately 14 weeks after the first infusion of immunotherapy. Patients fasted 6 hours before intravenous injection of approximately 3 MBq/kg of 18F-FDG. Following injection patients remained in a quiet room for approximately 60 minutes before acquisition. Patients were scanned from the top of the skull to the mid-thigh in the arms-down position except for patients with limb melanoma (limbs included).[14] Patients were allowed to breath normally during the PET and CT acquisitions (2 min per bed position). CT included injection of iodine in the absence of contraindications. PET data were acquired in three-dimensional (3D) mode and, for attenuation correction, were reconstructed using the CT data and followed by reconstruction using an ordered subsets expectation-maximization algorithm (True X PSF+TOF OSEM3D) into 200 × 200 matrices.
18F-FDG PET/CT imaging was performed prior to initiating immunotherapy (18F-FDG PET/CT 0), again after 4 or 6 cycles of ICI (4 for ipilimumab and pembrolizumab/ 6 for nivolumab) (18F-FDG PET/CT 1) (mean+/− SD: 13.82w ± 4.32), and sometimes during the follow-up especially to confirm an unconfirmed progressive disease (PET/CT 2) (mean delay between 18F-PET/CT 1 and 18F-PET/CT 2: +/− SD 11.19w ± 5.59). Mean time between initiation of ICI and 18F-FDG PET/CT 2 was 24.73 ± 9.53 weeks.
3. Data analysis
3.1. Response criteria
Datasets were analyzed using Syngo.via software by 2 experienced nuclear medicine physicians.
CT-based responses according to 18F-FDG PET/CT assessed by one physician were characterized according to RECIST1.1 [15] and iRECIST.[9]18F-FDG PET-based responses were evaluated by a second physician using PERCIST 1.0 [16] and PECRIT [10] criteria. PERCIST 1.0 was proposed as a new method for the quantitative assessment of metabolic changes in solid tumors and PECRIT criteria was recently proposed by Cho et al[10] for early prediction of eventual response to ICI therapy incorporating RECIST-based and PERCIST-based changes (change in peak SUV, normalized by lean body mass within a 1-cm3 spheric volume of interest, of the hottest lesion (SULpeak)) seen 3 to 4 weeks into treatment. Response criteria used in this study are summarized in Table 2.
Table 2.
Summary of Treatment Response Criteria.
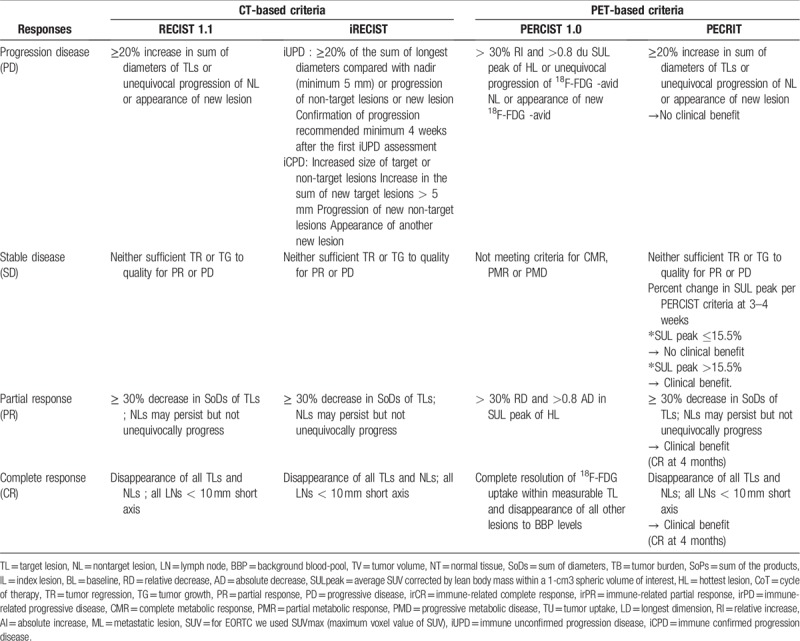
CT-based anti-tumor responses observed between PET/CT 0 and PET/CT 1 were classified as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) according to RECIST1.1 and iCR, iPR, iSD, iPD and immune unconfirmed progressive disease (iUPD) according to iRECIST.
18F-FDG PET-based anti-tumor responses were classified as complete metabolic response (CMR), partial metabolic response (PMR), stable metabolic disease (SMD) or progressive metabolic disease (PMD) according to PERCIST 1.0.
18F-FDG PET/CT-based responses were classified as clinical or no clinical benefit according to PECRIT criteria.
Based on 18F-FDG PET/CT results, 3 classes of treatment response were defined according to each criteria: i) Responder (R) which included respectively for RECIST1.1, iRECIST and PERCIST 1.0 criteria CR, PR, SD, iCR, iPR, iSD and CMR, PMR, SMD and non-PMD. ii) Non-Responder (NR) which included also for these 3 criteria: PD, iPD and PMD. iii) Undetermined (I): iUPD. These 3 classifications were also applied for PFS and OS.
Pseudoprogression was defined as a decrease or stabilization of the tumoral activity (or growth) of an initially evaluated disease progression.[17,18] Hyperprogression was defined as a RECIST 1.1 progression of ≥ 2-fold increase in tumor growth rate (TGR) at the first evaluation.[19]
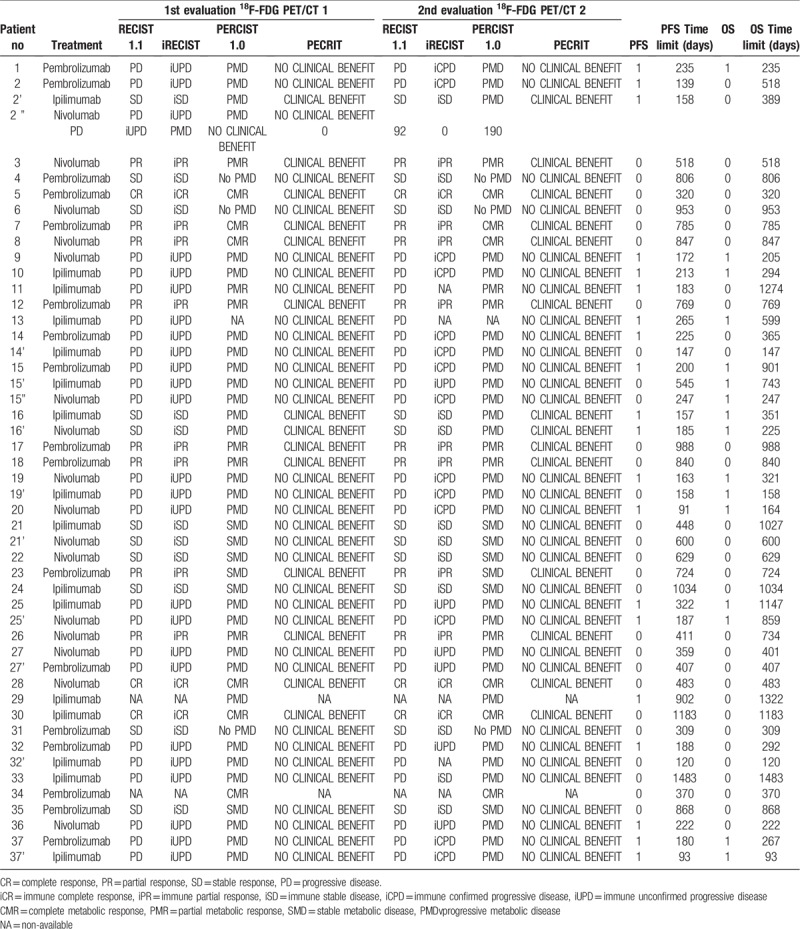
The duration of observation for each patient is included in Table 3.
Table 3.
Response assessments in 37 patients with metastatic melanoma receiving ICI therapies.
3.2. Follow-up
Patients were monitored through standard of care clinical and imaging examinations for assessment of PFS and OS regardless of 18F-FDG PET/CT data (PET/CT 0, PET/CT 1 and PET/CT 2). PFS was defined as the time from diagnosis to first reported disease progression or recurrence, or disease-related death. OS was defined as the time from diagnosis to death related to melanoma.
3.3. Clinical and biological data
Other criteria such as Eastern Cooperative Oncology Group (ECOG),[20] brain metastasis, ≥ 3 metastatic sites were also recorded.
3.4. Statistical analysis
3.4.1. PFS and OS were chosen as endpoints
Univariate analysis was performed to test the significance of the following factors: all response criteria and clinico-biological factors such as ECOG performance status >1, number of metastatic lesions ≥ 3, brain metastasis. The Kaplan–Meier method was used to estimate PFS and OS probabilities. A log-rank test was used to estimate survival distributions according to different interpretation criteria.
Significance level of P values was .05. All statistics were determined using XLSTAT-Life software (Addinsoft, Paris, France).
4. Results
4.1. Outcomes
Median PFS was 29.62 months (range 22.52–36.71) and median OS was 36.62 months (range 30.46–42.78). The overall results are summarized in Table 3.
According to RECIST 1.1, assessment of 35 evaluable scans (i.e., 47 imaging reviews) detected 3 CR, 8 PR, 11 SD and 25 PD. According to iRECIST, the conclusions were 3 iCR, 8 iPR, 11 iSD and 25 iUPD. Of these 18F-FDG PET/CT 2 findings, 14 iUPD were confirmed as iCPD progression (Fig. 1 ; patient 37 in Table 3). Of the 25 iUPD detected, 7 iUPD remained unchanged, 1 iUPD transformed into iSD, and 3 iUPD were not confirmed due to the change in therapy.
Figure 1.
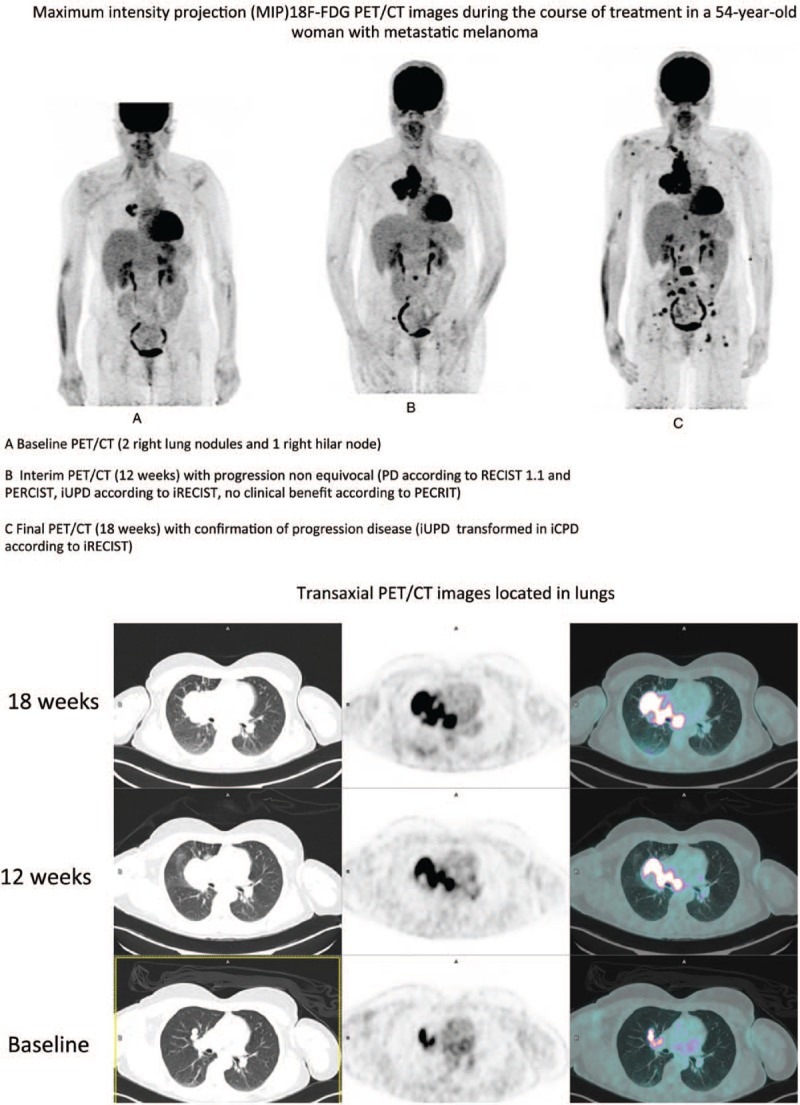
Maximum intensity projection (MIP)18F-FDG PET/CT images during the course of treatment in a 54-year-old woman with metastatic melanoma, A Baseline PET/CT (2 right lung nodules and 1 right hilar node), B Interim PET/CT (12 weeks) with progression non equivocal (PD according to RECIST 1.1 and PERCIST, iUPD according to iRECIST, no clinical benefit according to PECRIT), C Final PET/CT (18 weeks) with confirmation of progression disease (iUPD transformed in iCPD according to iRECIST).
According to PERCIST 1.0 criteria, therapeutic assessment of 36 evaluable scans (i.e., 48 imaging reviews) detected 6 CMR, 6 PMR, 6 SMD, 27 PMD and 3 non-PMD.
According to PECRIT criteria, therapeutic assessment of 35 evaluable scans (i.e., 47 imaging reviews) detected 14 clinical benefit and 33 no-clinical benefit.
4.1.1. Evaluation of criteria for progression-free survival (PFS)
According to RECIST 1.1, the median PFS were respectively 15.9, 25.6, 19.8, and 6.2 months for patients with CR, PR, SD, and PD.
According to iRECIST, the median PFS were respectively 15.9, 25.6, 20.2, 10.6 and 6.6 months for patients with iCR, iPR, iSD, iUPD, and iCPD.
According to PERCIST 1.0, the median PFS were respectively 20.9, 21.1, 22.2, 26.4, and 6.1 months for patients with CMR, PMR, SMD, non-PMD, and PMD.
According to PECRIT, median PFS was 20.41 months for patients with clinical benefit and 7.39 months for those with no clinical benefit.
Figure 2 displays Kaplan–Meier PFS results according to different treatment response criteria. Progression free survival estimates were statistically significant according to RECIST 1.1(P value = .001), iRECIST (P value < .0001) and PERCIST 1.0 criteria (P value = .000). The results were statistically non-significant according to PECRIT (P value = .072).
Figure 1 (Continued).
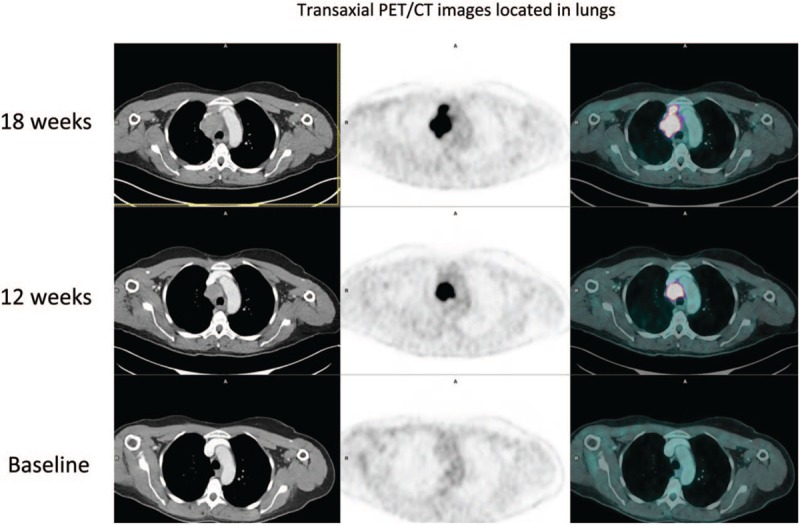
Maximum intensity projection (MIP)18F-FDG PET/CT images during the course of treatment in a 54-year-old woman with metastatic melanoma, A Baseline PET/CT (2 right lung nodules and 1 right hilar node), B Interim PET/CT (12 weeks) with progression non equivocal (PD according to RECIST 1.1 and PERCIST, iUPD according to iRECIST, no clinical benefit according to PECRIT), C Final PET/CT (18 weeks) with confirmation of progression disease (iUPD transformed in iCPD according to iRECIST).
This trend was also observed among responders and non-responders. According to RECIST 1.1, median PFS were respectively 22.24 and 6.18 months for responders and non-responders (P value < .0001).
According to iRECIST, median PFS were respectively 23.8, 10.58, and 6.57 months for responders, undetermined and non-responders (P value < .0001).
According to PERCIST 1.0, median PFS were respectively 23.80 and 6.14 months with responders and non-responders (P value < .0001).
4.1.2. Evaluation of criteria for overall survival (OS)
According to RECIST 1.1, the median OS were respectively 15.87, 25.54, 27.51, and 11.27 months for patients with CR, PR, SD, and PD.
According to iRECIST, the median OS were respectively 15.87, 25.54, 28.53, 11.39, and 9.66 months for patients with iCR, iPR, iSD, iUPD, and iCPD.
According to PERCIST 1.0, the median OS were respectively 20.84, 26.44, 28.53, 26.49, and 11.04 months for patients with CMR, PMR, SMD, non-PMD, and PMD.
According to PECRIT, median OS were respectively 24.70 and 17.03 months for patients with clinical benefit and no clinical benefit (P value = .082).
Figure 3 displays Kaplan–Meier OS results according to different treatment response criteria. Overall survival estimates were statistically significant according to RECIST 1.1 (P value = .005), iRECIST (P value < .0001), PERCIST 1.0 criteria (P value = .001). The results were statistically non-significant according to PECRIT (P value = .082).
Figure 2.
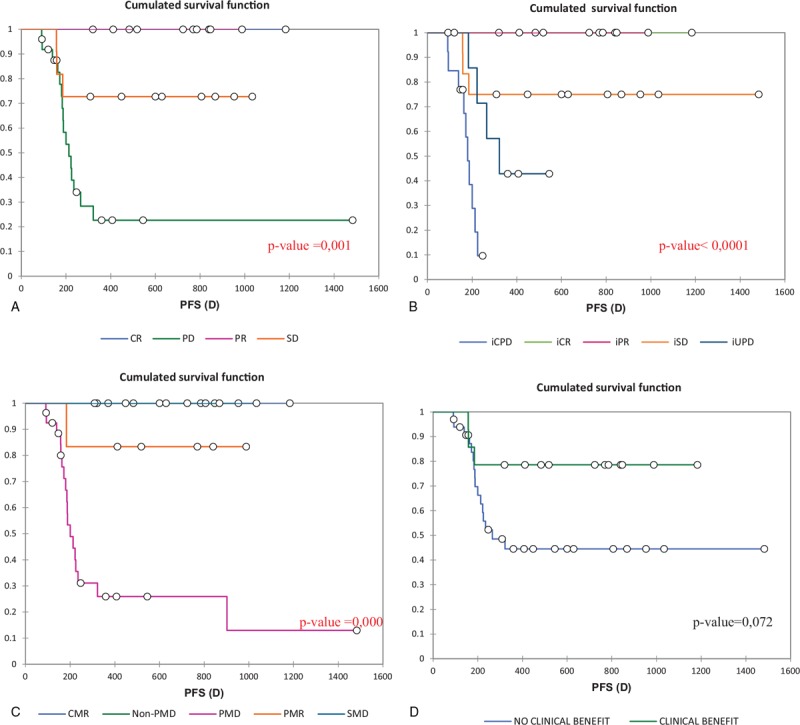
Kaplan–Meier progression free survival (PFS) estimates according to the different criteria, A RECIST 1.1, B iRECIST, C PERCIST 1,0, D PECRIT, CR = complete response, PR = partial response, SD = stable response, PD = progressive disease, iCPD = immune confirmed progressive disease, iCR = immune complete response iPR = immune partial response, iSD = immune stable disease, iUPD = immune unconfirmed progressive disease, CMR = complete metabolic response, PMR = partial metabolic response, SMD = stable metabolic response PMD = progressive metabolic disease.
This trend was also observed among responders and non-responders.
According to RECIST 1.1, median OS were respectively 25.8 and 11.27 months for responders and non-responders (P value = .000).
According to iRECIST, median OS were respectively 26.15,11.39, and 9.66 months for responders, undetermined and non-responders (P value < .0001).
According to PERCIST 1.0, median OS were respectively 26.15 and 11.04 months with responders and non-responders (P value < .0001).
4.1.3. Pseudo-progression and hyperprogression
In this cohort, only 1 patient had a pseudoprogression (patient 33 in Table 3), a 67-year-old male treated with ipilimumab had progression in an initial left lung lesion and a lower diaphragmatic node at the first assessment (18F-FDG PET/CT 1) according to iRECIST after 15 weeks. No confirmation of disease progression at 18F-FDG PET/CT 2 after 19 weeks. iUPD observed at the first assessment was transformed into iSD according to iRECIST criteria (Fig. 4 ). No hyperprogression was reported in this study.
Figure 3.
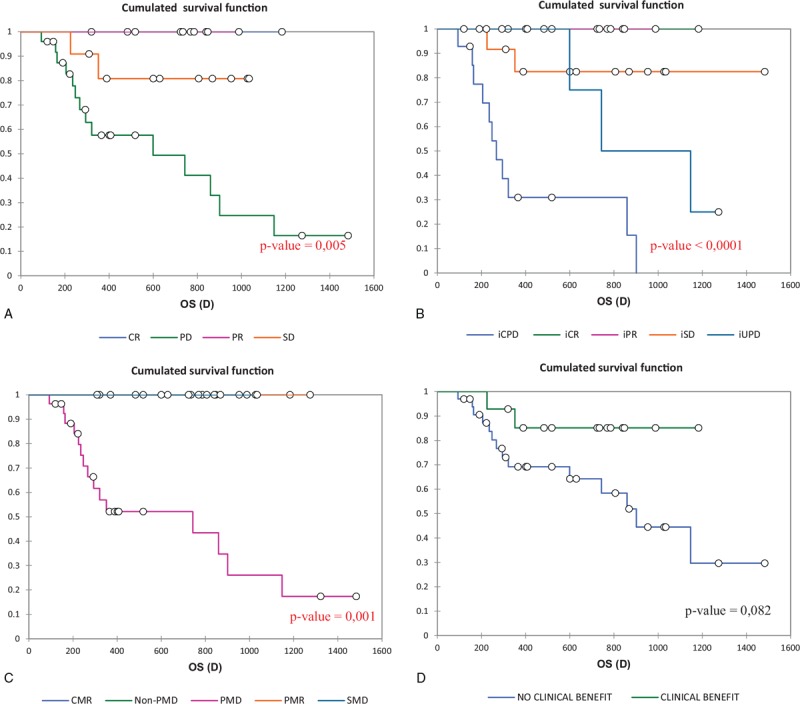
Kaplan-Meier overall survival (OS) estimates according to the different criteria, A RECIST 1.1, B iRECIST, C PERCIST 1,0, D PECRIT.
Figure 4.
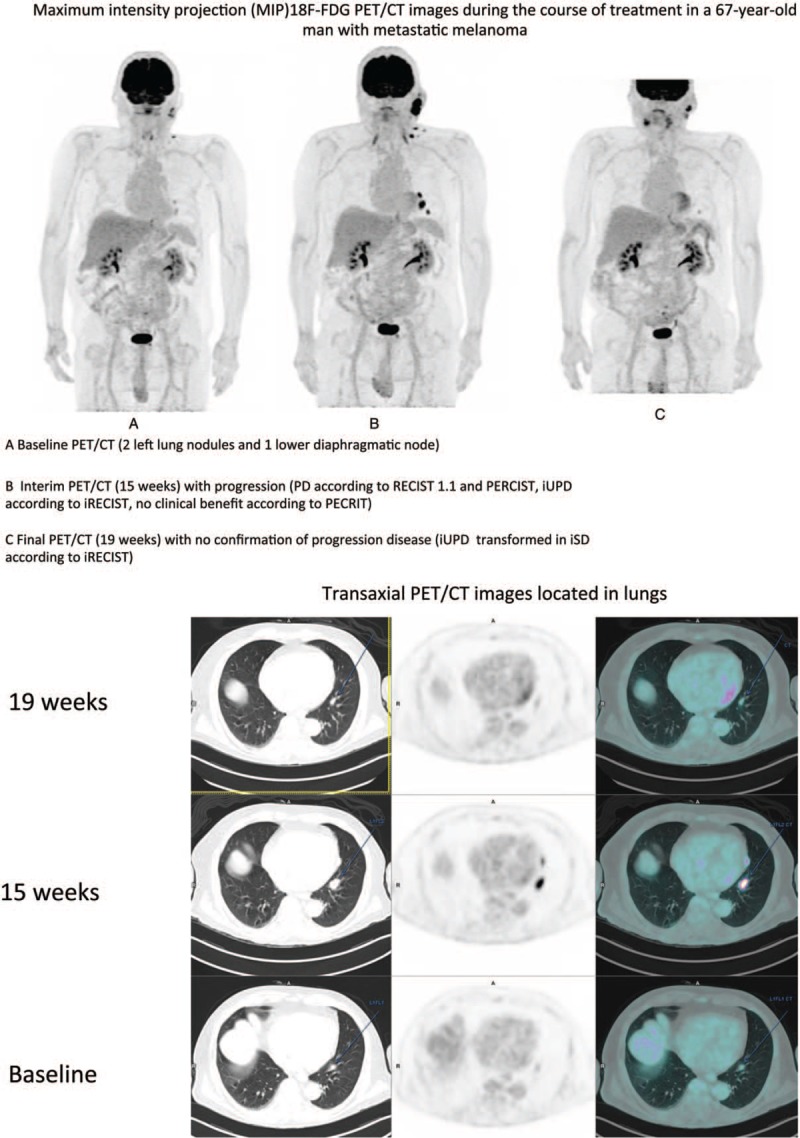
Maximum intensity projection (MIP)18F-FDG PET/CT images during the course of treatment in a 67-year-old man with metastatic melanoma, A Baseline PET/CT (2 left lung nodules and 1 lower diaphragmatic node), B Interim PET/CT (15 weeks) with progression (PD according to RECIST 1.1 and PERCIST, iUPD according to iRECIST, no clinical benefit according to PECRIT), C Final PET/CT (19 weeks) with no confirmation of progression disease (iUPD transformed in iSD according to iRECIST).
Figure 4 (Continued).
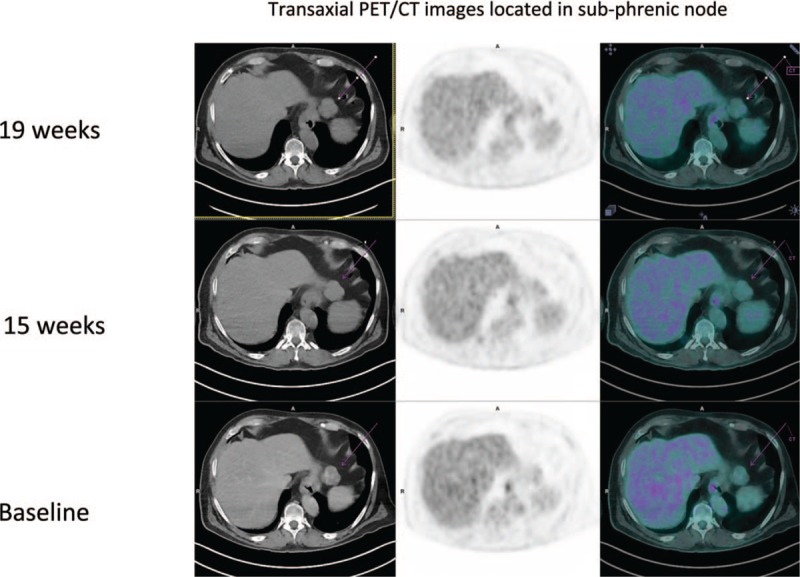
Maximum intensity projection (MIP)18F-FDG PET/CT images during the course of treatment in a 67-year-old man with metastatic melanoma, A Baseline PET/CT (2 left lung nodules and 1 lower diaphragmatic node), B Interim PET/CT (15 weeks) with progression (PD according to RECIST 1.1 and PERCIST, iUPD according to iRECIST, no clinical benefit according to PECRIT), C Final PET/CT (19 weeks) with no confirmation of progression disease (iUPD transformed in iSD according to iRECIST).
Clinical and biological data with poor prognosis [21–23] were also tested for PFS and OS with statistically non-significant results. The results are summarized in Table 4.
Table 4.
Univariate analysis of clinico-biological factors with poor prognosis present before the beginning of treatment.

5. Discussion
Immune checkpoint blockade agents represent a major advancement in cancer therapy and in particular in treatment of melanoma. These agents have demonstrated evidence of benefits on overall survival and patient safety.[5,7,24,25] The prognosis of melanoma has improved significantly since the advent of immunotherapy by ICI with survival in 40% of patients after 3 years, and allows prolonged complete responses even after stopping treatment.[5,26] Most cancer treatment response evaluations are based on CT-changes in tumor size according to morphological criteria such as RECIST 1.1.[15,27] However, immunotherapy (e.g., ICI) response assessment challenges the conventional measurements and criteria used for changes in tumor response (e.g., size and volume). In addition to the challenges of irregularly shaped or morphologically complex tumors, ICI agents mechanism of action, which depends on host's adaptive immune system,[28–30] can generate unusual response patterns, for example, pseudoprogression,[17,18] hyperprogression[19], atypical and delayed responses. Clinical trials on ICI were developed and conducted in late 2000s for the first time in treatment of melanoma. These clinical trials used RECIST1.1 criteria for assessment of response to treatment. The drawback with conventional criteria such as RECIST1.1, is the incapacity to classify unconventional and additional tumor response patterns to immunotherapeutic agents. Consequently, since 2009, other morphological criteria have been developed such as immune-related response criteria (irRC)[8], irRECIST[31–33] and iRECIST.[9] However, the design and methodology of the studies conducted to develop the above criteria did not provide a high level of evidence to reach consensual criteria for immune-related tumor response assessment. Common language has been reached in prediction of Hodgkin lymphoma and diffuse large B-cell lymphoma response to therapy.[34,35] In the rise of promising novel immunotherapeutic agents, it is of high importance to reach consensual criteria for better assessment of ICI agents effectiveness in melanoma.
In an attempt to reach consensual criteria, we retrospectively analyzed serial 18F-FDG PET/CT imaging according to morphological (RECIST 1.1, iRECIST) and functional (PERCIST 1.0, PECRIT) criteria to predict clinical response to therapy (i.e., Progression Free Survival (PFS) and Overall Survival (OS)) in patients with advanced melanoma receiving immune checkpoint blocking agents.
18F-FDG PET/CT is particularly interesting as an imaging modality for detection of response to ICI therapy, that is, metabolic changes in melanoma before morphological modifications. Similar to human neoplastic growth, inflammatory/infectious processes have high intracellular glucose metabolism. This could explain in patients with stable disease, ICI-related inflammatory response, that is, increased FDG uptake at an early PET/CT imaging, likely to demonstrate disease regression.[10,36]
Two morphological (RECIST 1.1 and iRECIST) and 1 functional (PERCIST 1.0) criteria predicted response to ICI therapy. PECRIT clinical benefit vs no-clinical benefit survival results were not significantly different.
In the prospective study of Cho et al, 20 patients with advanced melanoma receiving ICI underwent 18F-FDG PET/CT at three time points: prior to treatment initiation (SCAN-1), at days 21–28 (SCAN-2), and at 4 months (SCAN-3). Tumor response at post-treatment SCAN-2 and SCAN-3 was assessed according to RECIST 1.1, immune-related response criteria (irRC), PERCIST 1.0 and European Organization for Research and Treatment of Cancer (EORTC). The authors demonstrated that 18F-FDG-PET/CT scans performed early in ICI therapy could predict eventual response. RECIST 1.1, irRC, PERCIST 1.0 and EORTC criteria demonstrated respective 75%, 70%, 70%, and 65% accuracy in predicting best overall response.[10] So Cho et al suggested the use of PECRIT criteria, a combination of functional and anatomical parameters obtained from PET/CT 3 to 4 weeks after therapy onset, for early prediction of eventual response to ICI therapy. According to Cho et al study, patients with stable disease by RECIST1.1 at 3 to 4 weeks, an increase > 15.5% in SULpeak of the hottest lesion by 18F-FDG PET/CT was associated with eventual clinical benefit (PR or CR at 4 months or SD ≥ 6 months). Their PECRIT sensitivity, specificity and accuracy to predict response at 4 months were 100%, 93.3%, and 95.0% respectively. The discrepancy observed for PECRIT between our study (no significant results for PFS and OS when using PECRIT) and that of Cho et al may be explained by the delay to perform interim 18F-FDG PET/CT (3–4 weeks for Cho et al, 14 weeks in our study).
Sachpekidis et al found in 22 patients with metastatic melanoma treated by ipilimumab that assessment by PERCIST 1.0 criteria after 2 cycles of ipilimumab is highly predictive of the final treatment outcome in patients with PMD and SMD. Our PERCIST 1.0 results were consistent with the above literature, that is, predictive of treatment response[11].
In our study, only 1 pseudo-progression confirmed (2.85%) was described, which is lower compared to the rate reported in Hodi et al study which found 12% of pseudoprogressors among patients treated by pembrolizumab[15,18] and a rate of 6.7% in the study of Chiou et al also in a cohort of patients treated by pembrolizumab.[37] No hyperprogression was diagnosed in our study. Our lower % of pseudo-progression can probably be explained by the fact that 3 of our patients (i.e., classified as iUPD on 18F-FDG PET/CT1), did not have the 18F-FDG PET/CT2 due to treatment change and also by the frequency of tumor assessment.
To our knowledge, this is the first study comparing PECRIT a new 18F-FDG PET/CT criteria, with other validated criteria (PERCIST 1.0, RECIST 1.1, iRECIST) for prediction of ICI treatment response in metastatic melanoma.
Nevertheless, our study has some limitations. First, it was carried out in a single center with relatively small cohort. In addition, morphological criteria RECIST 1.1 and iRECIST were assessed on 18F-FDG PET/CT not on dedicated CT. Nevertheless, approximately 70% 18F-FDG PET/CT were performed with iodine injection as CT dedicated but without sequential acquisition. Response assessment time should be shorten with interim PET/CT performed after 4 to 8 weeks after ICI onset to detect easier atypical responses as pseudo-progression or hyperprogression.
The retrospective nature of the study provides a non-homogeneous methodology.
Our study suggests that 18F-FDG PET/CT scans could detect eventual ICI-response in patients with metastatic melanoma. According to our study, iRECIST and PERCIST 1.0 may provide the most optimal ICI-related response classification.
6. Conclusion
Based on survival analyses, 2 morphological (RECIST 1.1, iRECIST) and 1 functional criteria (PERCIST 1.0) appeared significantly predictive of PFS and OS in patients with unresectable metastatic melanoma treated by ICI. The novel functional PECRIT criteria did not seem to be suitable in late (>3–4 weeks) assessment of ICI-response classification. These preliminary results warrant further validation in larger cohort prospective studies using an early (within 3–4 weeks) treatment assessment time-point.
Author contributions
Conceptualization: Olivier Delcroix, Pierre-Yves Salaun, Ronan Abgral, Solène Querellou.
Data curation: Karim Amrane, Solène Querellou.
Formal analysis: Ronan Abgral, Nathalie Keromnes, Solène Querellou.
Investigation: Delphine Le Goupil, Gilles Quere, Sylvie Gouva.
Methodology: Gilles Quere, Ronan Abgral, Solène Querellou.
Supervision: Gilles Quere, Solène Querellou.
Validation: Gilles Quere, Solène Querellou.
Visualization: Solène Querellou.
Writing – original draft: Karim Amrane, Solène Querellou.
Writing – review & editing: Gilles Quere, Ulrike Schick, Ronan Abgral, Zarrin Alavi, Solène Querellou.
Olivier Delcroix orcid: 0000-0001-6157-222X.
Footnotes
Abbreviations: 18F-FDG PET/CT = 18F-FluoroDeoxyGlucose Positron Emission Tomography/Computed Tomography, 3D = three-dimensional, BRAFi = BRAF inhibitor, CMR = complete metabolic response, CR = complete response, CT = computed tomography, ECOG = Eastern Cooperative Oncology Group, EORTC = European Organization for Research and Treatment of Cancer, I = undetermined, ICI = immune checkpoint inhibitors, iCR = immune Complete Response, iPD = immune progressive disease, iPR = immune partial response, irRC = immune-related response criteria, iSD = immune stable disease, iUPD = immune unconfirmed progressive disease, MEKi = MEK inhibitor, MRI = magnetic resonance imaging, NR = non-responder, NSCLC = non-small cell lung cancer, OS = overall survival, PD = progressive disease, PFS = progression free survival, PMD = progressive metabolic disease, PMR = partial metabolic response, PR = partial response, R = responder, SD = stable disease, SMD = stable metabolic disease, TGR = tumor growth rate.
Prediction of response to Immune Checkpoint Inhibitor Therapy using 18F-FDG PET/CT in patients with melanoma.
The authors have no funding and conflicts of interest to disclose
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Cancer Facts & Figures 2017. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. [Google Scholar]
- [3].Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–0. [DOI] [PubMed] [Google Scholar]
- [4].Bagcchi S. Pembrolizumab for treatment of refractory melanoma. Lancet Oncol 2014;15:e419. [DOI] [PubMed] [Google Scholar]
- [5].Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- [6].Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375–84. [DOI] [PubMed] [Google Scholar]
- [8].Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res Off J Am Assoc Cancer Res 2009;15:7412–20. [DOI] [PubMed] [Google Scholar]
- [9].Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cho SY, Lipson EJ, Im H-J, et al. Prediction of response to immune checkpoint inhibitor therapy using early time-point FDG-PET/CT imaging in patients with advanced melanoma. J Nucl Med 2017;jnumed.116.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sachpekidis C, Larribere L, Pan L, et al. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging 2015;42:386–96. [DOI] [PubMed] [Google Scholar]
- [12].Sachpekidis C, Anwar H, Winkler J, et al. The role of interim 18F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging 2018;45:1289–96. [DOI] [PubMed] [Google Scholar]
- [13].Seith F, Forschner A, Schmidt H, et al. 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur J Nucl Med Mol Imaging 2018;45:95–101. [DOI] [PubMed] [Google Scholar]
- [14].Querellou S, Keromnes N, Abgral R, et al. Clinical and therapeutic impact of 18F-FDG PET/CT whole-body acquisition including lower limbs in patients with malignant melanoma. Nucl Med Commun 2010;31:766–72. [DOI] [PubMed] [Google Scholar]
- [15].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl 19902009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [16].Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009;50:122S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer Oxf Engl 19902018;88:38–47. [DOI] [PubMed] [Google Scholar]
- [18].Hodi FS, Hwu W-J, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016;34:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920–8. [DOI] [PubMed] [Google Scholar]
- [20].Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- [21].Recommandations, scores, échelles, fiches patients - Recommandations - Société Française de Dermatologie. http://www.sfdermato.org/recommandations-scores-et-echelles/recommandations.html. [Google Scholar]
- [22].Shivers SC, Wang X, Li W, et al. Molecular staging of malignant melanoma: correlation with clinical outcome. JAMA 1998;280:1410. [DOI] [PubMed] [Google Scholar]
- [23].Dessureault S, Soong SJ, Ross MI, et al. Improved staging of node-negative patients with intermediate to thick melanomas (>1 mm) with the use of lymphatic mapping and sentinel lymph node biopsy. Ann Surg Oncol 2001;8:766–70. [DOI] [PubMed] [Google Scholar]
- [24].Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- [26].Robert C, Ribas A, Hamid O, et al. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol 2016;34:9503–19503. [Google Scholar]
- [27].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- [28].Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci 2008;105:3005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ribas A, Comin-Anduix B, Economou JS, et al. Intratumoral immune cell infiltrates, FoxP3, and Indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res 2009;15:390–9. [DOI] [PubMed] [Google Scholar]
- [30].Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013;19:3936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nishino M, Gargano M, Suda M, et al. Optimizing immune-related tumor response assessment: does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer 2014;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bohnsack O, Hoos A, Ludajic K. 1070P: Adaptation of the immune related response criteria: irrecist. Ann Oncol 2014;25:iv369–1369. [Google Scholar]
- [34].Meignan M, Gallamini A, Meignan M, et al. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk Lymphoma 2009;50:1257–60. [DOI] [PubMed] [Google Scholar]
- [35].Van Heertum RL, Scarimbolo R, Wolodzko JG, et al. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: an operational approach for clinical trials. Drug Des Devel Ther 2017;11:1719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ribas A, Benz MR, Allen-Auerbach MS, et al. Imaging of CTLA4 blockade-induced cell replication with 18F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med 2010;51:340–6. [DOI] [PubMed] [Google Scholar]
- [37].Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015;33:3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


