Abstract
Since the standard reconstructive option after large segmental resection of proximal humeral tumors remained controversial, we designed and applied plate-prosthesis composite (PPC) for this circumstance. The purposes of the study were to: compare the functional outcome, implant survival (IS), surgical risk of PPC with those of conventional proximal humeral prosthesis (PHP); and describe the design and reconstructive procedure of PPC.
Twenty patients (11 males, 9 females), who received intraarticular proximal humeral resection without preservation of abductor mechanism, were included in this study, with a mean resection length accounting for 72.5% (range, 61.9–81.8%) of whole humeral length. According to the reconstructive options, we categorized patients into PPC group (9 patients) and PHP group (11 patients). PPC was a semi-custom-made endoprosthesis, with modular proximal part same as PHP and custom-made distal part including dumpy stem and composite lateral anatomic plate for distal humerus. The mechanical prosthetic complication was defined as the imaging evidence regardless of reoperation. The IS was defined as the time from surgery to the occurrence of mechanical prosthetic complication.
The mean follow-up time was 40.1 months (range, 14–129). The Musculoskeletal Tumor Society 93 scores of PPC and PHP group showed no significant difference (73.3% vs 70.0%, P = .46). Compared to PHP group, PPC group showed significantly lower mechanical prosthetic complication rates (0 vs 45.4%, P = .03) and better IS (86.0 vs 59.3 ± 21.7 months, P = .028). Moreover, the comparison of surgical time (3.2 vs 3.3 hours, P = .60), blood loss (288.9 vs 376.4 mL, P = .15) and perioperative complication rates (11.1% vs 18.2%, P = .58) between 2 groups showed no differences.
For reconstruction after large segmental resection of proximal humeral tumors, PPC achieved better IS while maintained similar functional outcome compared to conventional PHP without influencing the complexity and safety of surgery.
Keywords: bone neoplasms, endoprosthesis, functional reconstruction, plate-prosthesis composite, proximal humerus
1. Introduction
The proximal humerus is one of the most common sites of bony neoplasms.[1] Segmental resection of the tumor bearing bone and the enclosed soft tissues is imperative in primary bone sarcoma and is performed increasingly in patients with solitary bone metastasis to reduce tumor burden and improve oncologic outcome. Afterwards, functional reconstruction is indispensable to provide a platform for elbow and hand function and to restore shoulder function.[2] Among the various reconstructive options, endoprosthesis replacement is probably the most widely used option because of its availability, relatively low complication rate, high implant survival (IS), and comparable functional results to those of other approaches.[3–5]
However, in circumstance of large segmental resection of proximal humeral tumor, the endoprosthetic reconstruction is arduous because of the limited residual humerus which precludes the fixation of prosthetic stem and increases the risk of implant failure.[6] In literatures, reconstructive options in such instance were mainly nonendoprosthetic methods, which, however, led to unsatisfactory outcome with comparatively poor function and/or high complication rates.[6–9] As a result, the standard reconstructive option for massive proximal humeral tumors remains controversial. Therefore, we designed and applied a plate-prosthesis composite (PPC) for reconstruction after large segmental resection of proximal humeral tumors in order to achieve low risk of implant failure while maintain acceptable functional outcome.
The aims of this study were to: compare the functional outcome, IS, surgical risk of PPC with those of conventional proximal humeral prosthesis (PHP); and describe the design and reconstructive procedure of PPC.
2. Materials and methods
This study was carried out after obtaining an approval from the hospital institutional review board committee. From a prospective database, we retrospectively reviewed 329 consecutive patients with proximal humeral tumors who underwent surgical treatment in our center during July 2003 to April 2017. The inclusion criteria included: received segmental resection of proximal humerus; the length of residual humerus, which was defined as the distance from the level of osteotomy to the superior border of olecranon fossa, was <10 cm; the bone defect was reconstructed by PPC or PHP. The exclusion criteria included: the glenoid was involved, requiring extraarticular resection; and incomplete clinical and/or follow-up data.
According to the inclusion and exclusion criteria, 309 patients were excluded due to meeting at least one of the following conditions: intralesional excision surgery (65 patients), length of residual humerus ≥10 cm (268 patients), using other reconstructive methods (97 patients), glenoid involvement (54 patients), and incomplete data (87 patients). Twenty patients who met the inclusion and exclusion criteria were enrolled in this study, including 11 males and 9 females with an average age of 24.3 years (range, 8–65) who underwent surgeries in our center during August 2007 and February 2017 (Table 1).
Table 1.
Demographic and surgical information of 20 patients.
All patients were diagnosed by biopsy before therapies. Standard neoadjuvant chemotherapy was performed for patients with osteosarcoma and Ewing sarcoma/PNET. The range of intraosseous involvement and soft-tissue mass was determined by preoperative X-ray, computed tomography (CT), and magnetic resonance imaging. The resection length was documented as the distance between greater tuberosity of humerus and level of osteotomy.
2.1. Operative technique
All patients received intraarticular proximal humeral segmental resection, and the attachment of deltoid muscle and rotator cuff on humerus of all patients was sacrificed because of the massive tumor involvement (Malawer Type IB[2]). The humeral shaft was transected at least 2 cm distal to the inferior extent of tumor. All tumors were widely resected, and the surgical margins were all negative. The glenoids in all patients remained intact. The mean resection length was 20.6 cm (range, 13–27 cm), which accounted for 72.5% (range, 61.9–81.8%) of total humeral length in average, and the mean length of residual humerus was 7.6 cm (range, 5.5–9.0 cm) (Table 1).
For reconstruction after large segmental resection of proximal humeral tumor, PHP represented as the only option till March 2011; after March 2011, surgeons recommended that all patients who required large segmental resection should receive PPC reconstruction while informing the patients clearly that the value of PPC in prevention of mechanical prosthetic complication was uncertain, and additional reconstructive procedures might prolong surgical time, increase blood loss, lead to unexpected complications, as well as increase cost of surgery. The decision for using PPC was discussed and codetermined by patients and surgeons with the patients’ consent. Meanwhile, the process of decision making on using the synthetic mesh for soft-tissue reconstruction was similar to that on using PPC.
According to the reconstructive options, we categorized patients into 2 groups: PPC (9 patients) and PHP (11 patients) group. The reconstructive technique in PHP group was to use a conventional modular endoprosthesis that was fixed to the host humerus by cement. The reconstructive technique in PPC group was to use a semi-custom-made PPC, of which the proximal modular component was as same as that of PHP while the stem was custom made to be a dumpy shape based on the length of residual humerus and the diameter of residual medullary cavity. Three transverse screw holes were predrilled on the distal part of the prosthetic body. The hole distances were designed as same as that of the preselected lateral anatomic plate for distal humerus, and the direction of holes were designed to assure that the plate fit the bone surface while the prosthesis cemented into the residual humerus at 35° of retroversion with respect to the forearm. During reconstruction, the prosthetic stem was firstly cemented into the residual humerus at 35° of retroversion with respect to the forearm, the plate was then fixed to prosthesis and residual humerus by locking screws (the diameter was 3.5 mm). Synthetic mesh was used in 15 patients (75.0%) for soft-tissue reconstruction, including 5 patients in PPC group and 7 patients in PHP group[10] (Table 1).
2.2. Follow-up
All patients were followed up clinically and radiologically every 3 months in the 1st year after surgery, and every 6 to 12 months afterwards. The functional outcome was evaluated during the final follow-up using the Musculoskeletal Tumor Society (MSTS) 93 system for the upper extremity.[11]
According to the failure mode classification for tumor endoprostheses reported by Henderson, we categorized mechanical prosthetic complications into 3 types: type 1, soft-tissue complication, including instability, dislocation/subluxation, tendon rupture or aseptic wound dehiscence; type 2, aseptic loosening, referring to clinical and radiographic evidence of loosening; type 3, structural complication, including periprosthetic or prosthetic fracture, osteolysis, and deficient osseous supporting structure.[12] In this study, the mechanical prosthetic complication was defined as the imaging evidence of above-mentioned complications regardless of reoperation. The IS was defined as the time from surgery to the occurrence of mechanical prosthetic complication.
2.3. Statistical analyses
Statistical analysis was performed using the Statistical Package for the Social Science (SPSS) software version 19.0 (SPSS Inc, Chicago, IL). The overall survival (OS), disease-free survival (DFS) and IS were calculated using the Kaplan–Meier method and were compared by log-rank test. Nonparametric test and Mann–Whitney U test were used for comparing age, resection length, the percentage of resection length in total humeral length, length of residual humerus, surgical time, volume of intraoperative hemorrhage, follow-up time, and MSTS 93 scores in 2 groups. Fisher exact test was used to compare the distribution of gender and histologic diagnosis, the rates of using synthetic mesh for soft-tissue reconstruction, death rates, disease progression rates, and mechanical prosthetic complication rates in 2 groups. P < .05 was considered significant.
3. Results
The mean surgical time and intraoperative hemorrhage of all 20 patients were 3.3 hours (range, 1.5–6.5) and 337.0 mL (range, 150–500 mL), respectively. There was no perioperative death. Perioperative complications showed in 3 patients (15.0%), all of which were radial nerve injuries. The mean follow-up time was 40.1 months (range, 14–129). Eight patients (40.0%) were dead and mean OS was 68.1 ± 14.6 months; disease progression was found in 8 patients (40.0%) with a mean DFS on 73.1 ± 15.4 months, including 7 patients with pulmonary metastases and 1 patient with pulmonary metastasis and local recurrence sequentially (Fig. 1).
Figure 1.
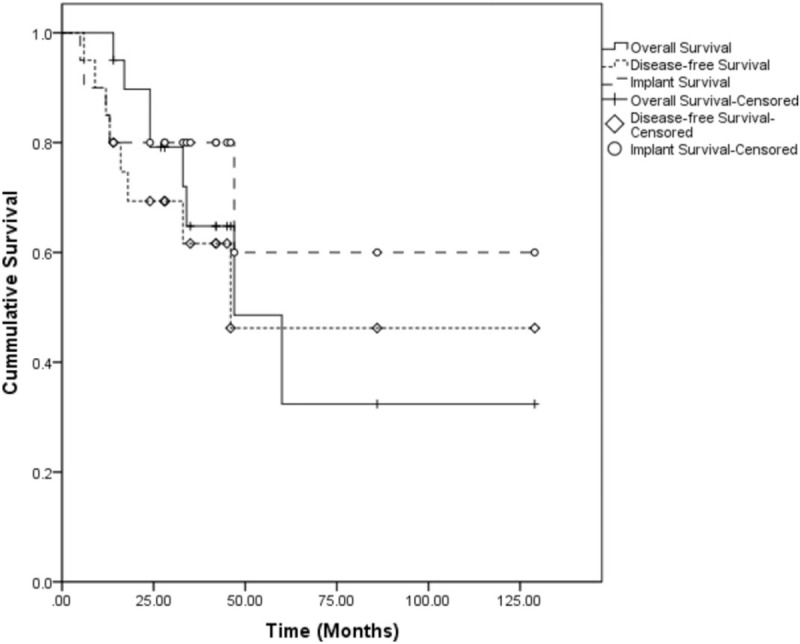
Kaplan–Meier curves showing overall survival, disease-free survival, and implant survival of 20 patients.
Imaging evidence of mechanical prosthetic complications emerged in 5 patients (25.0%), including 1 type 1 (subluxation), 1 type 2 (loosening of prosthetic stem) and 3 type 3 (2 cases of periprosthetic osteolysis and 1 case of periprosthetic fracture). For these patients, conservative treatments such as immobilization and avoiding weight-bearing were firstly applied to prevent further prosthetic failure. Among 5 patients, only 1 patient with type 2 mechanical prosthetic complication required reoperation, who did her rehabilitation not in conformity with physician's advice after the complication emerged 6 months postoperatively. She received revision operation 23 months after initial surgery and the endoprosthesis was replaced by an antibiotic-loaded bone cement spacer (Fig. 2). The mean IS according to our definition was 88.6 ± 16.8 months (Fig. 1). The mean postoperative MSTS 93 score was 71.5% (range, 60.0–90.0%). The MSTS 93 scores of patients who underwent soft-tissue reconstruction using synthetic mesh were better than those of patients who received no synthetic mesh reconstruction, whereas the difference showed no significant (72.4% vs 68.7%, P = .45).
Figure 2.
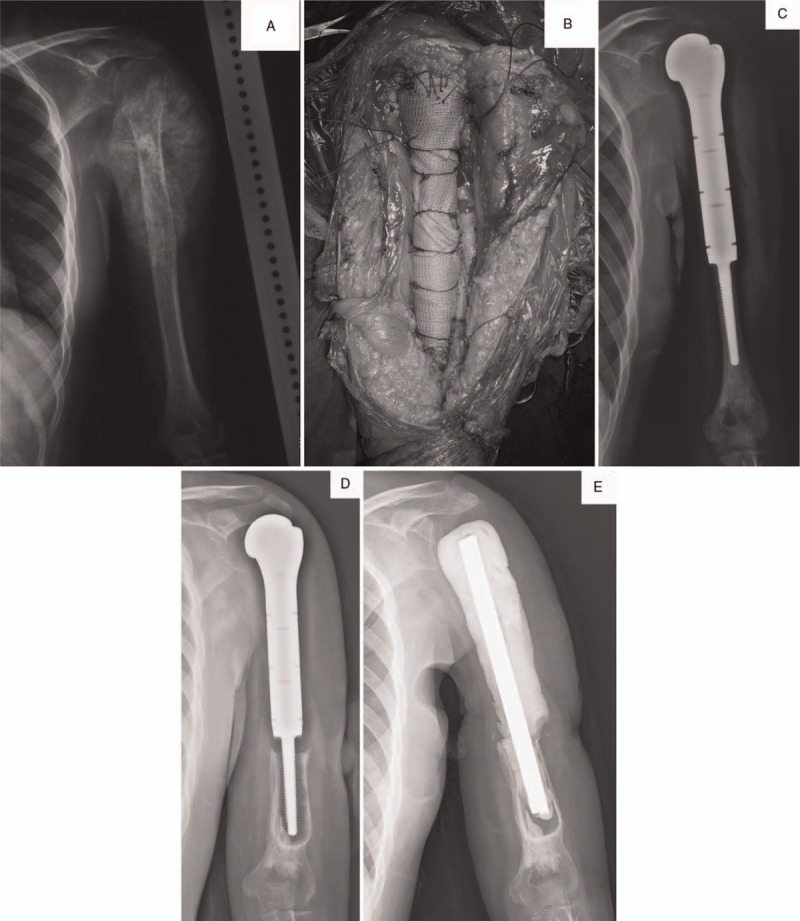
Female patient, 21 years old, left proximal humeral osteosarcoma. She received segmental resection of left proximal humerus. The resection length accounted for 69.1% of total humeral length and the residual humeral length was 8.5 cm. Proximal humeral prosthesis (PHP) was used for reconstruction. (A) Preoperative X-ray and magnetic resonance imaging. (B) The bone defect was reconstructed by PHP with a synthetic mesh used for soft-tissue reconstruction. (C) Postoperative X-ray. (D) X-ray on 23 months after surgery showed significant loosening (type 2) combined with periprosthetic fracture and osteolysis (type 3). (E) X-ray after reoperation.
The PPC reconstruction was used in 9 patients (45.0%), of which the mean resection length was 20.4 cm (range, 13–24.5 cm) accounting for 72.8% (range, 61.9–81.7%) of total humeral length, and the mean length of residual humerus was 7.4 cm (range, 5.5–9.0). Surgeries took 3.2 hours (range, 2.3–6.5) in average and the average volume of intraoperative hemorrhage was 288.9 mL (range, 150–500 mL). There was 1 patient showed clinical features of radial nerve injury in PPC group. After surgery, the mean MSTS 93 score was 73.3% (range, 60.0–90.0%) with no mechanical prosthetic complication (Fig. 3 ).
Figure 3.
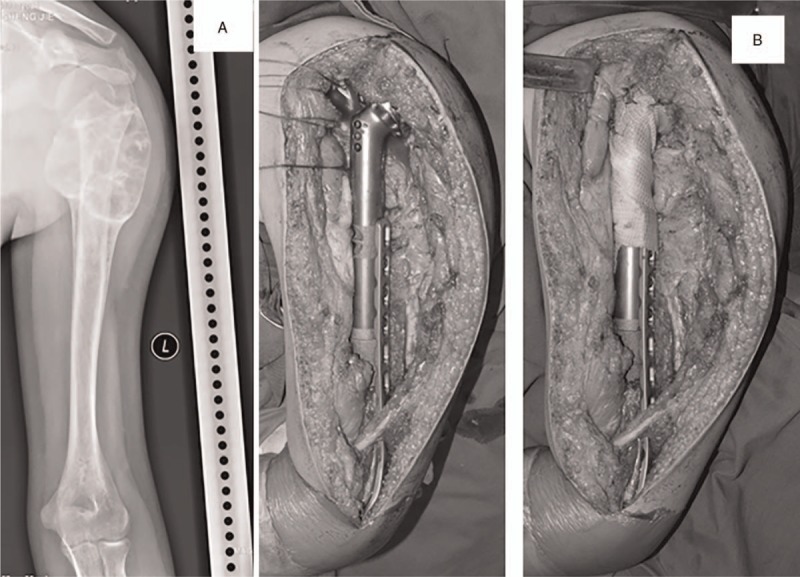
Male patient, 15 years old, left proximal humeral osteosarcoma. He received segmental resection of left proximal humerus. The resection length accounted for 71.4% of total humeral length and the residual length was 8 cm. Plate-prosthesis composite (PPC) was used for reconstruction. (A) Preoperative X-ray and magnetic resonance imaging. (B) PPC was used for reconstruction and a synthetic mesh was used for soft-tissue reconstruction. (C) Postoperative X-ray. (E) X-ray on 14 months after surgery showed no evidence of mechanical prosthetic complications.
The other 11 patients (55.0%) received conventional PHP reconstruction (Fig. 2). The baseline data, including age, distribution of gender and histologic diagnosis, resection length, the percentage of resection length in total humeral length, length of residual humerus, the rates of using synthetic mesh for soft-tissue reconstruction, follow-up time, death rates, OS (Fig. 4A), disease progression rates, and DFS (Fig. 4B), of PPC group was compared to that of PHP group, which resulted in no differences indicating that 2 groups were comparable (Table 2). Postoperatively, there was no difference of MSTS 93 scores between 2 groups (73.3% vs 70.0%, P = .46). However, PPC group showed a significantly lower mechanical prosthetic complication rate and better IS when compared to PHP group (0 vs 45.4%, P = .03; 86.0 vs 59.3 ± 21.7, P = .028) (Fig. 4C). Moreover, in concern of the safety of surgery, the comparison of surgical time (3.2 vs 3.3 hours, P = .60), blood loss (288.9 vs 376.4 mL, P = .15) and perioperative complication rates (11.1% vs 18.2%, P = .58) between 2 groups showed no differences (Table 2).
Figure 3 (Continued).
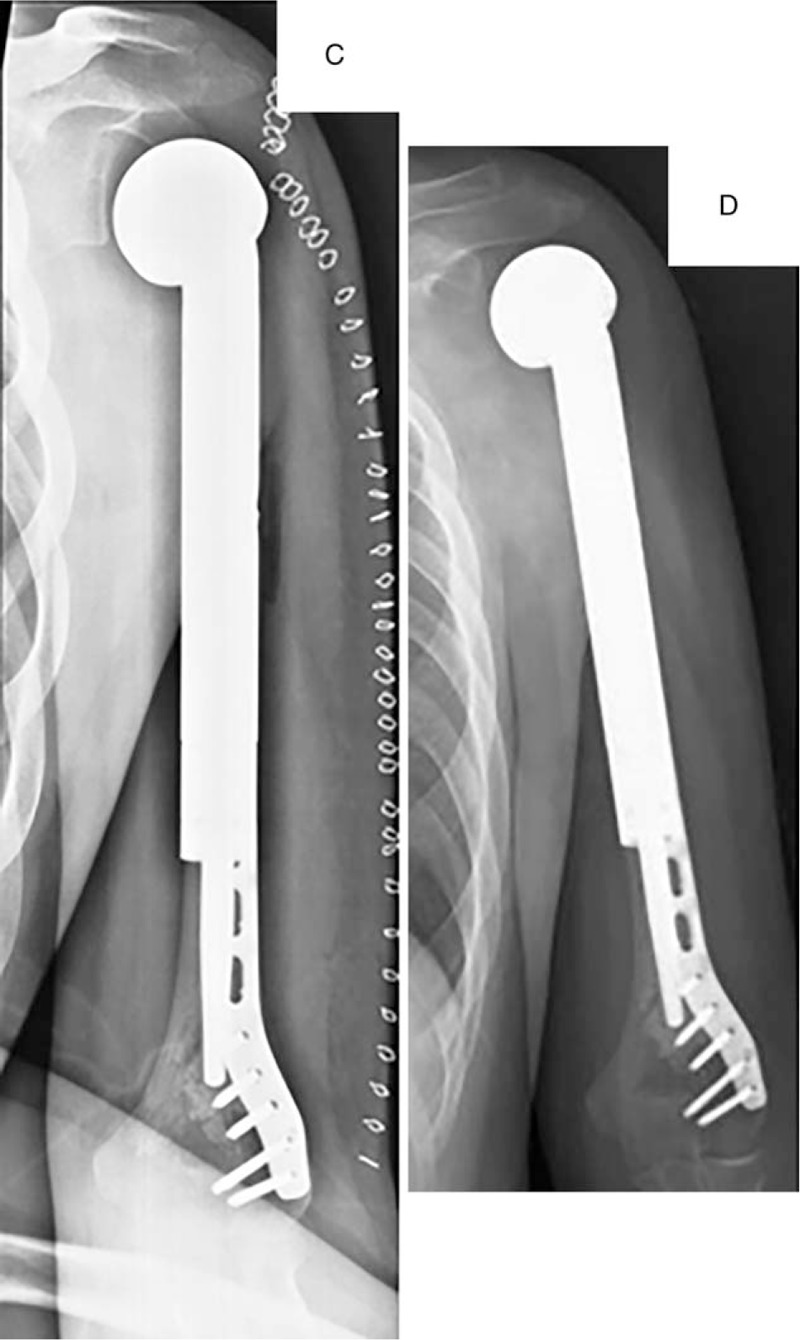
Male patient, 15 years old, left proximal humeral osteosarcoma. He received segmental resection of left proximal humerus. The resection length accounted for 71.4% of total humeral length and the residual length was 8 cm. Plate-prosthesis composite (PPC) was used for reconstruction. (A) Preoperative X-ray and magnetic resonance imaging. (B) PPC was used for reconstruction and a synthetic mesh was used for soft-tissue reconstruction. (C) Postoperative X-ray. (E) X-ray on 14 months after surgery showed no evidence of mechanical prosthetic complications.
Table 2.
The comparison between PPC and PHP group.
Figure 4.
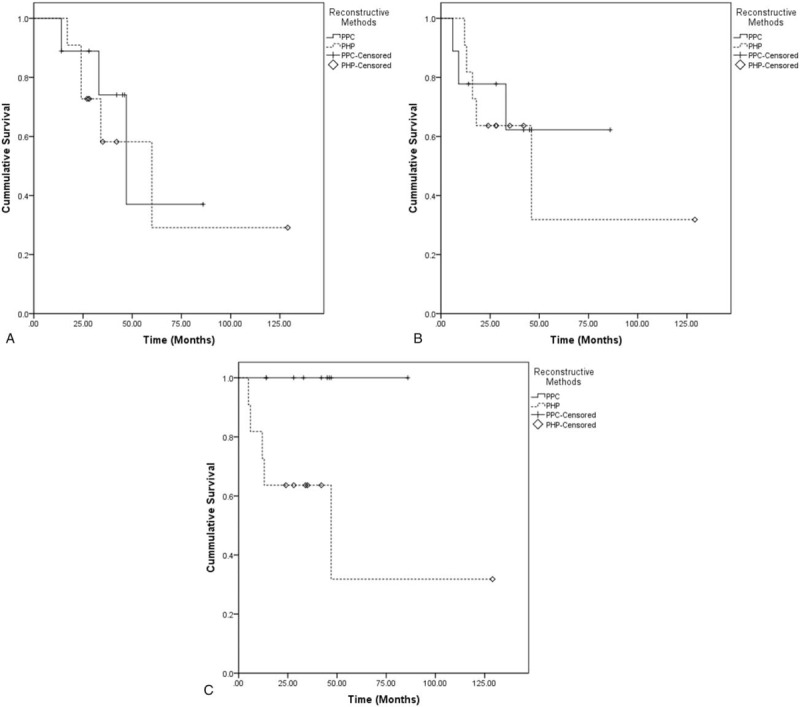
Kaplan–Meier curves showing comparison of (A) overall survival, (B) disease-free survival, and (C) implant survival between plate-prosthesis composite (PPC) and proximal humeral prosthesis (PHP) groups.
4. Discussion
There was a great diversity of options for reconstruction of regular bone defect after segmental resection of proximal humeral tumors, including endoprosthesis,[3,5,13,14] osteoarticular allograft,[7,8] allo/autograft prosthesis composite,[15–17] autograft (vascularized or nonvascularized fibula),[18,19] arthrodesis,[20] etc. Thereinto, endoprosthetic replacement is probably the most widely used because of its availability, relatively low complication rate, high IS, and comparable functional results by comparison with those of other approaches.[1,4,13] However, although the shoulder function could be preserved by the conventional PHP, it was unsatisfied because of damage of the abductor mechanisms and the unconstrained design of the prosthesis. Therefore, modified endoprosthetic reconstruction, for example reverse shoulder endoprosthesis,[16,21] using synthetic mesh for enhancing soft-tissue attachment,[10,22] etc, had been reported to improve the functional outcome. In this study, the overall functional outcome was consistent with that in literatures. Patients with soft-tissue reconstruction by synthetic mesh showed better MSTS 93 scores than patients without it, which, however, showed no significant difference that might due to the limited number of patients.
Nevertheless, in patients with extensive tumor involvement in the proximal humerus requiring large segmental resection, the fixation of PHP is formidable because of the short residual stump; even the prosthetic stem could be implemented, the intramedullary fixed length is significantly shortened, which may lead to loosening or fracture. Furthermore, the massive loss of tendon attachments resulted from large segmental resection impedes the shoulder function.[3,6] In this study, although the functional results were comparably acceptable, the mechanical prosthetic complication rates and IS were unfavorable, extremely in PHP group, which confirmed that large segmental resection for proximal humerus increased the risk of the implant failure of conventional endoprosthetic reconstruction.
There were few studies regarding the reconstructive option for large segmental resection of proximal humeral tumor, most of which were nonendoprosthetic reconstruction. Jamshidi et al described the outcome of using osteoarticular allograft, in which more than half of patients showed extensive tumor involvement of humerus (resection length >154.5 mm). The mean MSTS 93 score was 84.9% and the incidence of mechanical prosthetic complication which required reoperation, for example, fracture, osteolysis, and un-union, was 25%.[8] Barbier et al demonstrated that using clavicula pro humero technique for patients with a median resection length on 20 cm which accounted for 62.5% of total length of humerus. The median MSTS 93 score was 77% and complication rate reached up to 71.4%.[18] Puri and Gulia described a simple reconstructive method, using a custom-made plate fixed to residual humerus by screws and to glenoid by polypropylene mesh for reconstruction. The incidence of mechanical complications was 12.5%, but the function was restricted (MSTS 93 scores were not documented).[6] According to above literatures, it was unsatisfactory for nonendoprosthetic reconstructive options to reconstruct the massive proximal humeral bone defect because of the poor functional outcome and/or the unfavorable implant outcome, which was similar to the outcome of conventional endoprosthetic reconstruction.
To solve this problem, we designed PPC, that is, a modified PHP. The conceptual basis is to add extramedullary fixation to conventional PHP aiming at reinforcing the intrinsically intramedullary fixation. The biomechanical structure of PPC reconstruction resembles that of using plate for fixation of periprosthetic humeral fracture after proximal humeral endoprosthetic replacement.[23,24] In reconstruction after resection of tumor with extensive proximal humerus involvement, from functional point of view, PPC attained similar upper-limb function as PHP did, which was more promising than some of the nonendoprosthetic reconstructive options did.[6,9] In addition, comparing to PHP, even comparing to biologic reconstruction reported in literatures,[8,18] patients using PPC showed significantly superior IS, which might attribute to the combination of intra- and extramedullary fixation that could distinctly strengthen the fixation of prosthesis. Consequently, it can lower the risk of prosthetic loosening and periprosthetic fracture which mostly occurs in endoprosthetic reconstruction and eradicate circumvent bone fracture, osteolysis, and nonunion resulted from bone grafting which mostly emerges in biologic reconstruction. Moreover, in this study, the surgical time, intraoperative hemorrhage, and perioperative complication rate of PPC group showed no significant difference to those of PHP group, which indicated that reconstruction by PPC would not influence the complexity or safety of surgery. Therefore, we hold the opinion that PPC is the ideal endoprosthetic reconstructive option for large segmental resection of proximal humeral tumors, because of the acceptable functional outcome and the improved IS.
In addition to its retrospective design, our study has further limitations. Firstly, the sample size was limited, and the follow-up period was comparatively short, which might influence the reliability and validity of this study. A large, long-term, case–control study is warranted to validate the conclusion of this study. Secondly, the mechanical prosthetic complication rate and IS, especially in PHP group, were unsatisfactory compared to those reported in literatures, which might attribute to: above-mentioned insufficient residual humerus in patients included in this study which precluded the rigid fixation of conventional endoprosthesis; and the definition of endpoint of IS in this study, which was defined as the emergence of imaging evidence of mechanical prosthetic complication regardless of the revision surgery, was of inconformity with that in literatures, which were mostly reported as reoperation owing to the mechanical prosthetic complication.
5. Conclusion
For reconstruction after large segmental resection of proximal humeral tumors, PPC achieved better IS while maintained similar functional outcome compared to conventional PHP without impairing the complexity and safety of surgery. Using PPC for reconstruction could represent as the ideal reconstructive option after large segmental resection of proximal humeral tumors.
Author contributions
Conceptualization: Wei Guo.
Data curation: Ran Wei, Rongli Yang, Xiaodong Tang.
Formal analysis: Yi Yang, Tao Ji.
Investigation: Rongli Yang, Xiaodong Tang.
Methodology: Yi Yang, Tao Ji.
Software: Yi Yang, Tao Ji.
Supervision: Wei Guo.
Writing – original draft: Ran Wei.
Writing – review & editing: Wei Guo.
Footnotes
Abbreviations: CT = computed tomography, DFS = disease-free survival, IS = implant survival, MSTS 93 scores = Musculoskeletal Tumor Society 93 scores, OS = overall survival, PHP = proximal humeral prosthesis, PPC = plate-prosthesis composite, SPSS = Statistical Package for the Social Science.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Teunis T, Nota SP, Hornicek FJ, et al. Outcome after reconstruction of the proximal humerus for tumor resection: a systematic review. Clin Orthop Relat Res 2009;472:2245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Malawer MM, Meller I, Dunham WK. A new surgical classification system for shoulder-girdle resections. Analysis of 38 patients. Clin Orthop Relat Res 1991;33–44. [PubMed] [Google Scholar]
- [3].Kumar D, Grimer RJ, Abudu A, et al. Endoprosthetic replacement of the proximal humerus. Long-term results. J Bone Joint Surg Br 2003;85:717–22. [PubMed] [Google Scholar]
- [4].van de Sande MA, Dijkstra PD, Taminiau AH. Proximal humerus reconstruction after tumour resection: biological versus endoprosthetic reconstruction. Int Orthop 2011;35:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Raiss P, Kinkel S, Sauter U, et al. Replacement of the proximal humerus with MUTARS tumor endoprostheses. Eur J Surg Oncol 2010;36:371–7. [DOI] [PubMed] [Google Scholar]
- [6].Puri A, Gulia A. An inexpensive reconstruction method after resection in tumors of the proximal humerus with extensive involvement of the diaphysis. Int J Shoulder Surg 2010;5:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bus MP, van de Sande MA, Taminiau AH, et al. Is there still a role for osteoarticular allograft reconstruction in musculoskeletal tumour surgery? A long-term follow-up study of 38 patients and systematic review of the literature. Bone Joint J 2017;99-B:522–30. [DOI] [PubMed] [Google Scholar]
- [8].Jamshidi K, Najd-Mazhar F, Abolghasemzadeh Ahangar F, et al. The effect of cement augmentation and anteromedial plating on proximal humerus allograft reconstruction. J Orthop Sci 2017;22:69–74. [DOI] [PubMed] [Google Scholar]
- [9].Kundu ZS, Gogna P, Gupta V, et al. Proximal humeral reconstruction using nail cement spacer in primary and metastatic tumours of proximal humerus. Strategies Trauma Limb Reconstr 2013;8:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tang X, Guo W, Yang R, et al. Synthetic mesh improves shoulder function after intraarticular resection and prosthetic replacement of proximal humerus. Clin Orthop Relat Res 2015;473:1464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;241–6. [PubMed] [Google Scholar]
- [12].Henderson ER, Groundland JS, Pala E, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am 2011;93:418–29. [DOI] [PubMed] [Google Scholar]
- [13].Cannon CP, Paraliticci GU, Lin PP, et al. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg 2009;18:705–10. [DOI] [PubMed] [Google Scholar]
- [14].Mayilvahanan N, Paraskumar M, Sivaseelam A, et al. Custom mega-prosthetic replacement for proximal humeral tumours. Int Orthop 2006;30:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Abdeen A, Hoang BH, Athanasian EA, et al. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am 2009;91:2406–15. [DOI] [PubMed] [Google Scholar]
- [16].Lazerges C, Dagneaux L, Degeorge B, et al. Composite reverse shoulder arthroplasty can provide good function and quality of life in cases of malignant tumour of the proximal humerus. Int Orthop 2017;41:2619–25. [DOI] [PubMed] [Google Scholar]
- [17].Ruggieri P, Mavrogenis AF, Guerra G, et al. Preliminary results after reconstruction of bony defects of the proximal humerus with an allograft-resurfacing composite. J Bone Joint Surg Br 2011;93:1098–103. [DOI] [PubMed] [Google Scholar]
- [18].Barbier D, De Billy B, Gicquel P, et al. Is the clavicula pro humero technique of value for reconstruction after resection of the proximal humerus in children? Clin Orthop Relat Res 2017;41:2619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu T, Zhang Q, Guo X, et al. Treatment and outcome of malignant bone tumors of the proximal humerus: biological versus endoprosthetic reconstruction. BMC Musculoskelet Disord 2014;15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mimata Y, Nishida J, Sato K, et al. Glenohumeral arthrodesis for malignant tumor of the shoulder girdle. J Shoulder Elbow Surg 2015;24:174–8. [DOI] [PubMed] [Google Scholar]
- [21].Streitbuerger A, Henrichs M, Gosheger G, et al. Improvement of the shoulder function after large segment resection of the proximal humerus with the use of an inverse tumour prosthesis. Int Orthop 2015;39:355–61. [DOI] [PubMed] [Google Scholar]
- [22].Fujibuchi T, Matsumoto S, Shimoji T, et al. New endoprosthesis suspension method with polypropylene monofilament knitted mesh after resection of bone tumors in proximal humerus. J Shoulder Elbow Surg 2015;24:882–8. [DOI] [PubMed] [Google Scholar]
- [23].Kumar S, Sperling JW, Haidukewych GH, et al. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am 2004;86-A:680–9. [DOI] [PubMed] [Google Scholar]
- [24].Schoch B, Mehta S, Namdari S. Surgical fixation of periprosthetic humerus fractures using an extension plate: surgical technique and report of 5 cases. J Orthop Trauma 2017;31:e432–5. [DOI] [PubMed] [Google Scholar]


