Abstract
Helicobacter pylori (H. pylori) is the most prevalent chronic bacterial infection and is associated with chronic gastritis, peptic ulcer disease, and gastric adenocarcinoma. Although eradication therapy is widely performed for H. pylori infection, adverse events (AEs) are of particular concern in the elderly. This study investigated the efficacy and safety of H. pylori eradication therapy for elderly patients.
Retrospective investigation of 1271 cases (median age: 61 years, 730 male) of H. pylori infection was performed to compare clinical indications and outcomes among the younger group (<65 years old), elderly group (65–74 years old), and super-elderly group (>75 years old).
Chronic gastritis (77.0%) and gastric and/or duodenal ulcer (16.4%) were the most frequent indications for eradication therapy in the cohort. The respective eradication and AE rates for the first and second treatment regimens were 92.1% (1044 of 1133 cases) and 9.1% (103 of 1133 cases) and 84.2% (123 of 146 cases) and 8.9% (13 of 146 cases). No significant differences were detected for eradication rate or AE frequency between the super-elderly group and the other groups. Prior to therapy, the super-elderly group had significantly less frequent chronic gastritis than the other groups but more frequent gastric or duodenal ulcer and post-gastric cancer treatment (all P < .001), indicating a reluctance for clinicians to treat very old patients, possibly due to unfounded concerns of complications.
Triple therapy for H. pylori eradication is effective and safe, even for elderly patients.
Keywords: adverse events, elderly patients, eradication, Helicobacter pylori (H. pylori), safety
1. Introduction
Helicobacter pylori (H. pylori) was firstly reported by Warren and Marshall in 1983 as a gram-negative bacterium found on the luminal surface of the gastric epithelium.[1] Conservative estimates suggest that half of the world's population is infected with H. pylori,[2] although country-specific prevalences appear to decline with economic improvement. For example, 70 to 80% of Japanese adults born before 1950 were infected by the bacterium, versus 45% for those born between 1950 and 1960 and 25% for those born between 1960 and 1970.[3] This rapid fall has been attributed to Japan's post-war economic progress and ameliorations in sanitation. However, many elderly patients remain infected with H. pylori until receiving eradication therapy.
H. pylori is known to cause chronic gastritis, gastric and duodenal ulcers, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue (MALT) lymphomas.[4–6] In addition to symptomatic treatment, H. pylori eradication also appears to reduce the risk of gastric cancer,[7] especially metachronous gastric cancer,[8] and enhance gastric corpus atrophy grade improvement from baseline.[9] Therefore, all individuals with evidence of active H. pylori infection should be offered with eradication therapy in the absence of screening programs for asymptomatic cases.
Several antibiotic regimens for H. pylori eradication therapy have been confirmed as effective and safe. [10]The adverse events (AEs) of eradication therapy are usually mild, with fewer than 10% of patients halting treatment due to unwanted reactions.[11] However, AE rates have been as high as 50% for a particular triple therapy regimen.[11,12] The frequency of AEs induced by H. pylori eradication therapy in the elderly also remains unclear and possibly misunderstood. This study therefore investigated the efficacy and safety of H. pylori eradication therapy for elderly patients.
2. Patients and methods
2.1. Patients
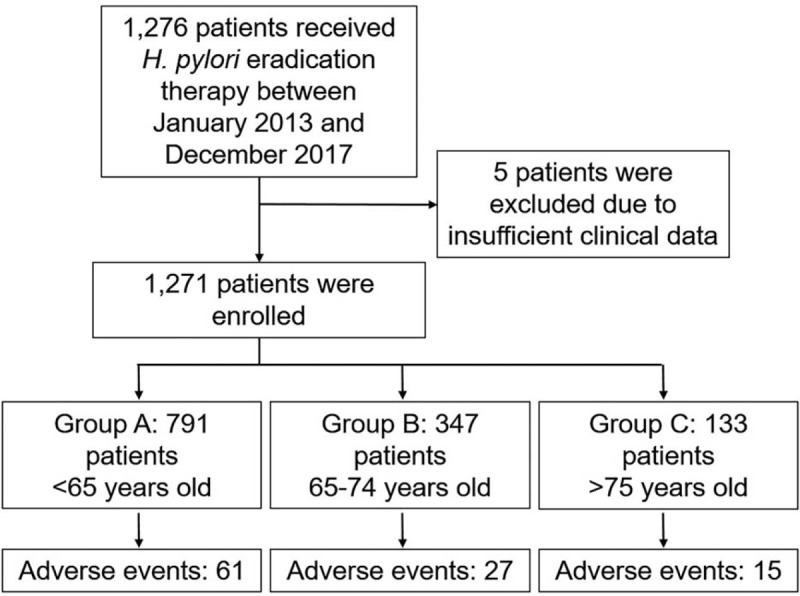
A total of 1276 patients who had received H. pylori eradication therapy between January 2013 and December 2017 were initially recruited in this retrospective, single-center study. After excluding cases lacking sufficient clinical data for analysis, 1271 patients were ultimately enrolled. The cohort was divided by age into the younger group (≤65 years), elderly group (65–74 years), and super-elderly group (≥75 years) based on the standards of the Japanese national health insurance system for comparisons of clinical characteristics including indication for eradication, eradication regimen, eradication rate, and rate and type of AEs.
2.2. Confirmation of H. pylori positivity and laboratory testing
H. pylori infection was confirmed by any positive result among serum anti-H. pylori antibodies, urea breath test, stool antigen test, and histopathology. All patients also underwent esophagogastroduodenoscopy (EGD) before eradication therapy to confirm the presence of active chronic gastritis.
Serum anti-H. pylori IgG antibody titer was measured using an enzyme immunoassay, with a level of >10 U/mL defined as positive (SRL, Inc., Tokyo, Japan). The urea breath test was performed using infrared spectroscopic analysis according to the manufacturer's instructions (BML, Inc., Tokyo, Japan). The stool antigen test was carried out using an enzyme-linked immunosorbent assay (SRL, Inc., Tokyo, Japan). Cases were histopathologically defined as H. pylori positive when bacterial bodies were evident in EGD-obtained tissue sections.
2.3. Treatment regimens for eradication
Patients were initially treated with a regimen of clarithromycin (CAM)-based triple therapy consisting of a proton pump inhibitor (PPI), amoxicillin (AMPC), and CAM for 7 days as a first line treatment. Unresponsive patients identified 4 to 8 weeks later by means of the urea breath test were immediately given PPI, AMPC, and metronidazole (MNZ) as a second regimen. Two-week regimens are currently not approved in Japan. Successful eradication was confirmed by a urea breath test performed 4 weeks or more after completion of triple antibiotic therapy.
2.4. Determination of AEs
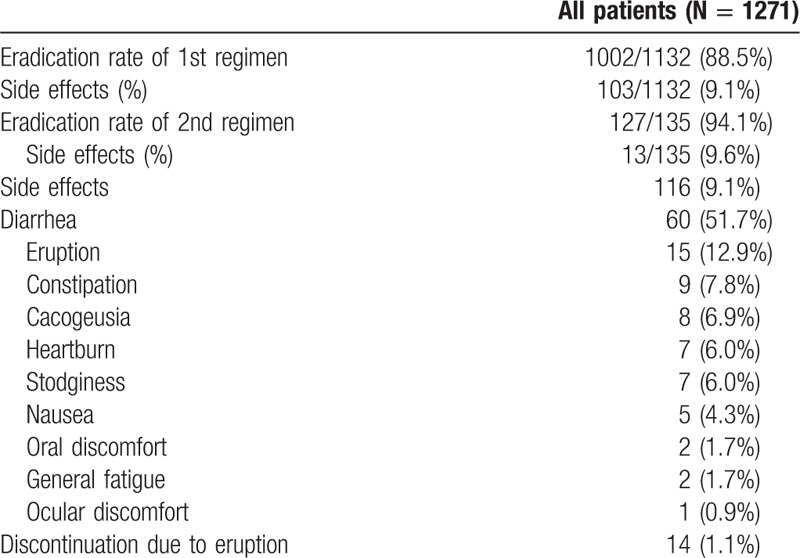
AEs including diarrhea, eruptions, and constipation (Table 2) were retrospectively determined by chart reviews of medical records.
Table 2.
Treatment outcomes and adverse events.
2.5. Statistical analysis
Statistical analysis was carried out using StatFlex ver. 7.0.2 (Artech Co., Ltd., Osaka, Japan). Data were presented as the median ± interquartile range for continuous variables, which were compared using the Mann–Whitney U test. Categorical variables were presented as the frequency (percentage) and analyzed using the chi-square test. All statistical tests were two-sided and evaluated at the 0.05 level of significance.
2.6. Ethics of the study
This investigation was reviewed and approved by the Institutional Review Board of Hokushin General Hospital (Nakano, Japan) (approval number: 2018021). As this was a retrospective observational study, written informed consent was not obtained from participating subjects, although study information was presented in an opt-out format at the hospital. This investigation was conducted in accordance with the principles of the 1975 Declaration of Helsinki as revised in 1983.
3. Results
3.1. Clinical characteristics of enrolled patients
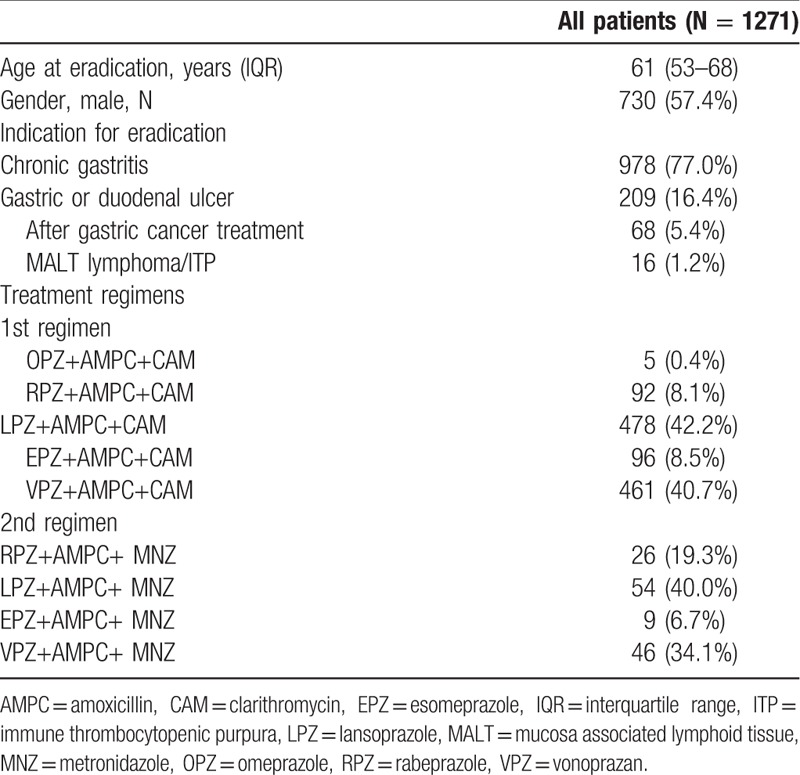
A flowchart of this study is shown in Fig. 1. The clinical characteristics of the enrolled patients are summarized in Table 1. Median age was 61 years and 57.4% of the cohort was male. Chronic gastritis (77.0%) was the most frequent indication for eradication therapy, followed next by gastric and/or duodenal ulcer with chronic gastritis (16.4%) and post-gastric cancer treatment with chronic gastritis (5.4%). The vast majority of patients were treated with lansoprazole + AMPC + CAM or vonoprazan + AMPC + CAM for the first line treatment (Table 1). The eradication rate of the first regimen was 92.1% (1044 of 1133 cases), with AEs seen in 9.1% (103 of 1133) of cases. The eradication rate of the second regimen was 84.2% (123 of 146 cases), with AEs recorded in 8.9% (13 of 146) of cases. The most frequently encountered AEs were diarrhea (51.7%), eruptions (12.9%), and constipation (7.8%). Fourteen patients halted treatment due to eruptions during the first regimen (Table 2).
Figure 1.
Study flowchart.
Table 1.
Clinical characteristics of enrolled patients.
3.2. Comparisons among age groups
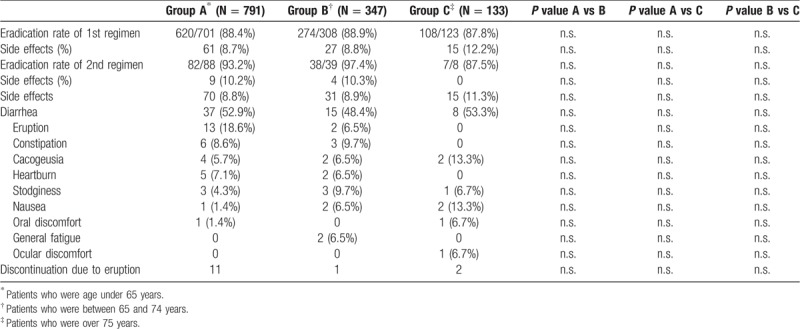
Clinical characteristics were compared among the younger, elderly, and super-elderly groups (Table 3). Super-elderly patients had a significantly less frequent indication of chronic gastritis than did the other groups (both P < .001) but more frequent indications of gastric or duodenal ulcer (both P < .001) and post-gastric cancer treatment (both P < .001). Apart from a significant difference between the younger and elderly groups for first eradication rate (P < .05), no remarkable differences were seen among the groups for the efficacy of either regimen. Moreover, no significant differences were observed in comparisons of AE rates among the groups (Table 4). The incidence of AEs was comparable between the first and second regimens.
Table 3.
Clinical characteristics of the three groups.
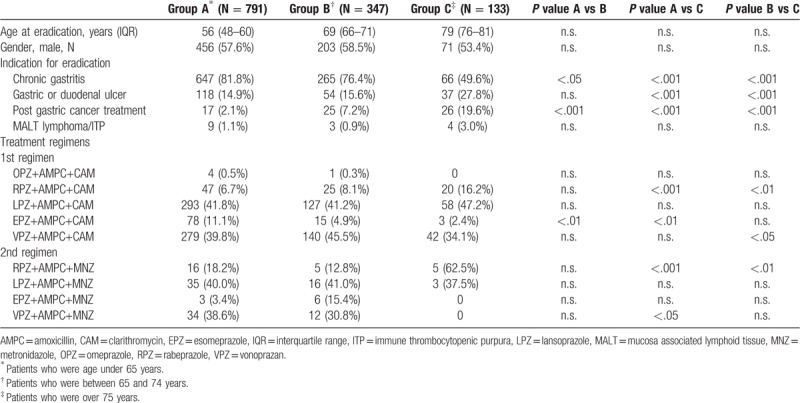
Table 4.
Treatment results of the three groups.
4. Discussion
This study uncovered two clinically significant points:
1. the eradication rates of H. pylori for very elderly patients were not inferior to those of younger patients, and
2. the AE rates of eradication therapy were comparable among all age groups.
Our findings confirm that eradication therapy is effective and safe for H. pylori, even for patients at an advanced age.
We divided the cohort of this retrospective study into three groups (<65 years old, 65–75 years old, and >75 years old) based on the age tiers established by the Japanese national health insurance system. Patients with active H. pylori infection were indicated for treatment, with the vast majority receiving eradication therapy due to chronic gastritis associated with chronic infection. Interestingly, less than half of the super-elderly patients had undergone eradication therapy, which indicated a possible reluctance among clinicians to prescribe such treatment. One reason might have been out of consideration for patient age and a higher perceived risk of complications. However, this study revealed no differences between the super-elderly and other groups in terms of eradication rates and AEs, suggesting that clinicians need not withhold treatment strictly based on age.
The infection route of H. pylori remains unknown, although person-to-person transmission through fecal/oral or oral/oral exposure seems most likely.[13,14] Person-to-person transmission has been supported by intrafamilial clustering of H. pylori infection, in which infected individuals were more likely to have infected spouses and children than were uninfected individuals.[15,16] Accordingly, our data highlight the importance of elderly or super-elderly patients who are infected with H. pylori to receive eradication therapy for prevention of cross-infection to younger uninfected family members, especially in multigenerational households.
As expected, the frequency of AEs in our cohort was <10%. The most frequent culprits, diarrhea and constipation, are manageable with drugs and probiotics. It was reported that supplementation with probiotics was effective not only in decreasing eradication therapy-related AEs, but also in increasing eradication rate.[17] Thus, H. pylori eradication triple therapy along with probiotics might improve both patient treatment and comfort.
This study has several limitations. First, it was conducted as a single-center cohort study of approximately 1000 patients. Secondly, due to its retrospective design, we cannot exclude the possibility that only elderly patients with a favorable systemic condition received eradication therapy.
5. Conclusions
Triple therapy for H. pylori eradication was effective and safe for elderly patients. Further studies are needed to examine the longer-term clinical effects of eradication therapy, such as the prevention of peptic ulcer recurrence, in larger, multi-center, elderly cohorts.
Acknowledgments
The authors would like to thank Trevor Ralph for his English editorial assistance.
Author contributions
Conceptualization: Satoshi Kobayashi.
Data curation: Satoshi Kobayashi, Satoru Joshita.
Formal analysis: Satoshi Kobayashi.
Methodology: Satoshi Kobayashi, Chikara Yamamoto.
Project administration: Satoshi Kobayashi, Satoru Joshita.
Resources: Satoshi Kobayashi, Chikara Yamamoto, Takumi Yanagisawa, Takayuki Miyazawa, Megumi Miyazawa, Daisuke Kubota, Junichi Sato.
Software: Satoshi Kobayashi.
Supervision: Eiji Tanaka.
Validation: Satoshi Kobayashi, Satoru Joshita, Takeji Umemura.
Visualization: Satoshi Kobayashi, Satoru Joshita.
Writing – original draft: Satoshi Kobayashi, Satoru Joshita.
Writing – review & editing: Chikara Yamamoto, Takeji Umemura, Eiji Tanaka.
Satoru Joshita orcid: 0000-0002-6364-9654.
Footnotes
Abbreviations: AEs = adverse events, AMPC = amoxicillin, CAM = clarithromycin, EGD = esophagogastroduodenoscopy, H. pylori = Helicobacter pylori, MALT = mucosa-associated lymphoid tissue, MNZ = metronidazole, PPI = proton pump inhibitor.
Financial support: None.
Conflicts of interest: The authors declare no conflicts of interest.
References
- [1].Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983;1:1273–5. [PubMed] [Google Scholar]
- [2].Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am 2000;29:559–78. [DOI] [PubMed] [Google Scholar]
- [3].Asaka M, Kimura T, Kudo M, et al. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology 1992;102:760–6. [DOI] [PubMed] [Google Scholar]
- [4].Feder R, Posner S, Qin Y, et al. Helicobacter pylori-associated peptic ulcer disease: a retrospective analysis of post-treatment testing practices. Helicobacter 2018;23:e12540. [DOI] [PubMed] [Google Scholar]
- [5].Mccoll KE. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362:1597–604. [DOI] [PubMed] [Google Scholar]
- [6].Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–9. [DOI] [PubMed] [Google Scholar]
- [7].Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 2016;150:1113–24e5. [DOI] [PubMed] [Google Scholar]
- [8].Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 2018;378:1085–95. [DOI] [PubMed] [Google Scholar]
- [9].Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392–7. [DOI] [PubMed] [Google Scholar]
- [10].Fujioka T, Aoyama N, Sakai K, et al. A large-scale nationwide multicenter prospective observational study of triple therapy using rabeprazole, amoxicillin, and clarithromycin for Helicobacter pylori eradication in Japan. J Gastroenterol 2012;47:276–83. [DOI] [PubMed] [Google Scholar]
- [11].De Boer WA, Tytgat GN. The best therapy for Helicobacter pylori infection: should efficacy or side-effect profile determine our choice? Scand J Gastroenterol 1995;30:401–7. [DOI] [PubMed] [Google Scholar]
- [12].Fischbach LA, Van Zanten S, Dickason J. Meta-analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther 2004;20:1071–82. [DOI] [PubMed] [Google Scholar]
- [13].Perry S, De La Luz Sanchez M, Yang S, et al. Gastroenteritis and transmission of Helicobacter pylori infection in households. Emerg Infect Dis 2006;12:1701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Megraud F. Transmission of Helicobacter pylori: faecal-oral versus oral-oral route. Aliment Pharmacol Ther 1995;9Suppl 2:85–91. [PubMed] [Google Scholar]
- [15].Kivi M, Johansson AL, Reilly M, et al. Helicobacter pylori status in family members as risk factors for infection in children. Epidemiol Infect 2005;133:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bamford KB, Bickley J, Collins JS, et al. Helicobacter pylori: comparison of DNA fingerprints provides evidence for intrafamilial infection. Gut 1993;34:1348–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tong JL, Ran ZH, Shen J, et al. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007;25:155–68. [DOI] [PubMed] [Google Scholar]


