Figure 1.
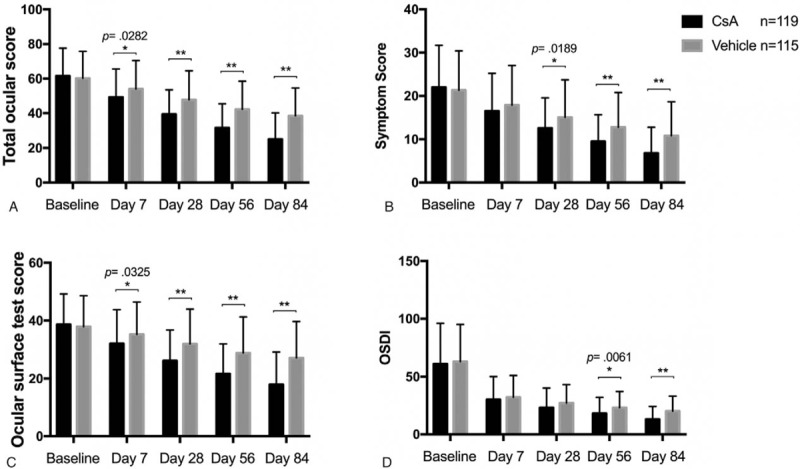
Comparison of the secondary endpoints within 84 days of randomized treatment with 0.05% CsA OE and vehicle controls in patients with moderate to severe dry eye disease. Values of total ocular score (A), symptom score (B), ocular surface test score (C) and OSDI (D) were displayed respectively. ∗P < .05, ∗∗P < .001, 2-tailed t test. CsA OE = cyclosporine ophthalmic emulsion, OSDI = ocular surface disease index.
