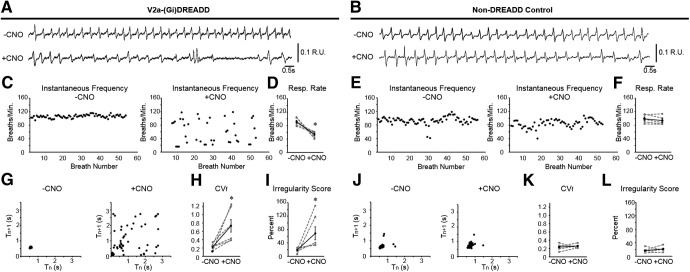
Figure 2.
Decreasing the excitability of V2a neurons causes irregular breathing in neonatal V2a-(Gi)DREADD mice. Representative traces of WBP before (top trace) and after (bottom trace) 10.0 mg/kg bw CNO treatment in a (A) V2a-Gi(DREADD) mouse and a (B) non-DREADD control (Chx10Cre/+) mouse at P2. C, E, Instantaneous respiratory frequency is plotted for at least 50 consecutive breaths in V2a-(Gi)DREADD mice and non-DREADD control mice before (left) and after (right) CNO treatment to illustrate the breath to breath consistency of respiratory frequency. D, F, Respiratory rate is decreased after silencing V2a neurons in V2a-(Gi)DREADD mice but remains unchanged following CNO treatment in non-DREADD control mice. Poincare maps generated by plotting the respiratory cycle period (Tn) versus the subsequent respiratory cycle period (Tn+1) from the same trials shown in C, E show an inconsistent period following CNO treatment in V2a-(Gi)DREADD mouse (G) but not in the non-DREADD control (J). H, CNO treatment (10.0 mg/kg bw) increases the CVf compared to baseline in V2a-(Gi)DREADD mice (p = 0.030, n = 6 but not in non-DREADD control mice (p = 0.619, n = 5; K). I, The average IS of the breathing frequency is increased in V2a-(Gi)DREADD mice following CNO (10.0 mg/kg bw; p = 0.031, n = 6) but remains unchanged in non-DREADD control mice (p = 0.363, n = 5; L). Gray dashed lines show individual animals. Solid black line shows the mean ± SE; *p < 0.05, paired t test or Wilcoxon signed-rank test (Fig. 1I).