Figure 5.
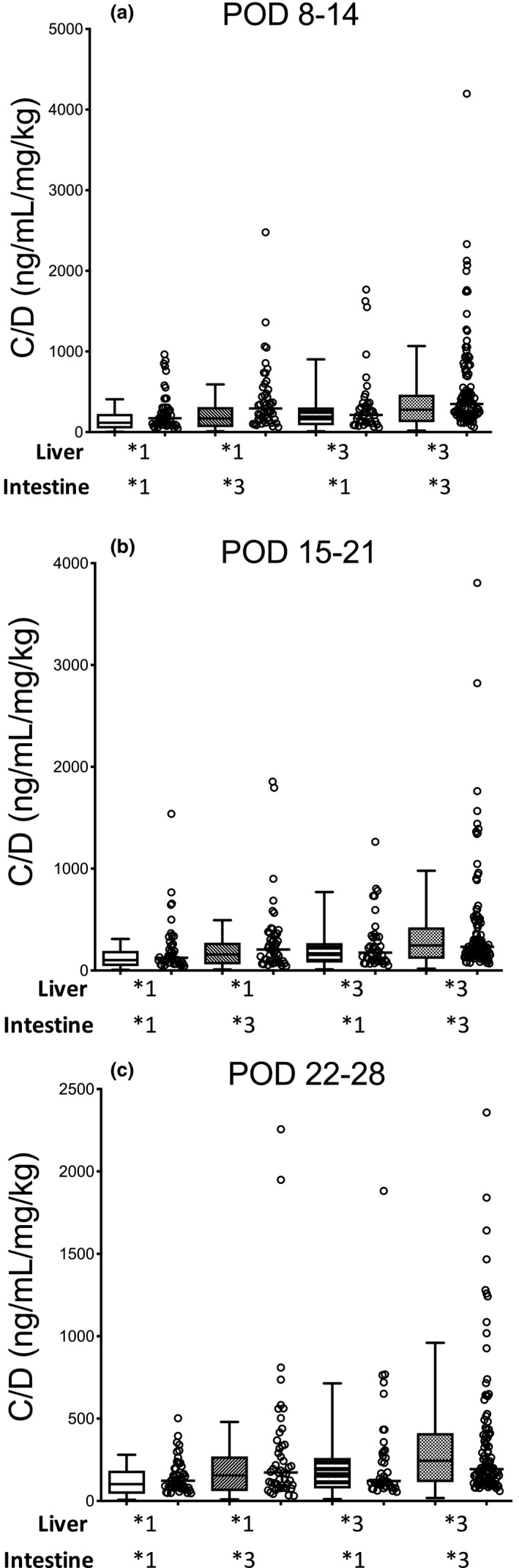
Comparison of the physiologically‐based pharmacokinetic–simulated and observed concentration of verification data divided by the concentration/dose (C/D) ratios of tacrolimus in each cytochrome P450 3A5 (CYP3A5) genotype combination of graft liver and small intestine for PODs (a) 8–14, (b) 15–21, and (c) 22–28 after living‐donor liver transplantation. Each box plot represents the interquartile range and 90% confidence interval of the predicted C/D ratio. Each open circle shows the observed C/D ratio, and each bar shows the median value. The number of patients with CYP3A5*1 allele in both the graft liver and small intestine, CYP3A5*1 allele in the graft liver and CYP3A5*3/*3 in the small intestine, CYP3A5*3/*3 in the graft liver and CYP3A5*1 allele in the small intestine, and CYP3A5*3/*3 in both the graft liver and small intestine were 54, 52, 47, and 119, respectively. POD, postoperative day.
