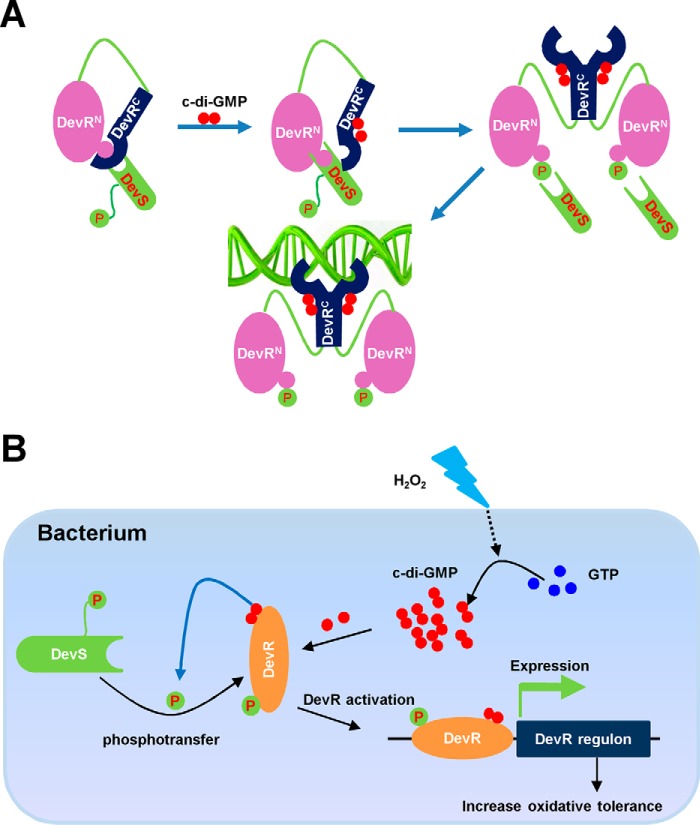
Figure 8.
Schematic representation of the mechanism on DevR activation by c-di-GMP and two-component system DevS/DevR and its regulation on mycobacterial tolerance to oxidative stress. A, DevR-activation mechanism co-triggered by c-di-GMP and DevS. DevRN dynamically interacts with DevRC and covers the phosphorylation site (Asp-54). Upon binding of c-di-GMP with the C-terminal domain of DevR (DevRC), its structure is rearranged, thereby disrupting the dynamic interaction between DevRC and DevRN. This allowed Asp-54 to be exposed. Therefore, DevS can transfer the phosphor group to Asp-54 of DevRN, which induced an interaction of DevRC with a second subunit to form an activated DevR dimer. B, model for c-di-GMP–triggered and DevS/DevR-dependent oxidative stress tolerance in mycobacteria. H2O2 induced c-di-GMP accumulation in mycobacteria, and then c-di-GMP bound to DevRC and refolded it to expose the phosphorylation site Asp-54, which enhanced the phosphotransfer between DevS and DevR and activated DevR. The activated DevR bound to the target promoter and up-regulated the expression of target genes to increase the survival rate of mycobacteria under oxidative stress.