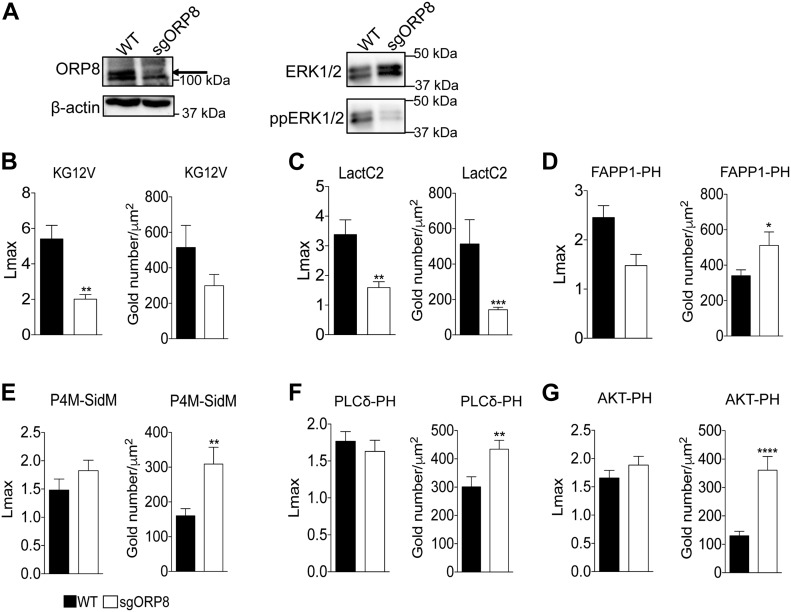
Figure 2. KRAS and PtdSer, PI4P, PIP2, and PIP3 clustering and membrane localization changes in OSBPL8 CRISPR KO CaCo-2 cells.
(A, B, C, D, E, F, G) CRISPR/Cas9 KO of ORP8 in CaCO-2 cells was validated by Western blotting and MAPK signaling assayed as ppERK levels in Western blots. Total ERK and β-actin levels were used as loading controls. PM sheets prepared from CaCO-2 parental (WT) and sgORP8 cells transiently transfected with GFP-KRASG12V (B), GFP-LactC2 (C), GFP-FAPP1-PH (D), GFP-P4M-SidM (E), GFP-PLCδ-PH (F), or GFP-AKT-PH (G) were labeled with anti-GFP antibodies coupled directly to 4.5-nm gold particles and visualized by EM. The amount of KRASG12V, LactC2, FAPP1-PH, P4M-SidM, PLCδ-PH, and AKT-PH on the PM was measured as gold particle labeling per μm2, and significant differences were quantified using t tests. Clustering of the GFP-tagged probes were quantified by univariate spatial analysis, summarized as Lmax values and significant differences were assessed using bootstrap tests (±SEM, n ≥ 12) (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.00001; KG12V: KRASG12V, LactC2: PtdSer probe, FAPP1-PH and P4M-SidM: PI4P probes, PLCδ-PH: PIP2 probe, AKT-PH: PIP3 probe).