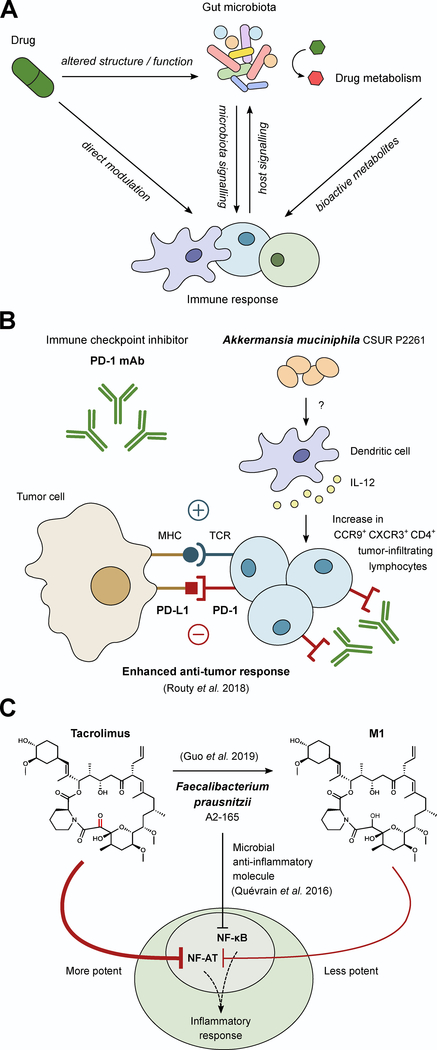
Figure 3. Microbial masters of immunomodulatory drugs.
(A) Due to the complex interplay between the immune system and the microbiota there are many potential ways microbes could alter the activity of immunomodulatory drugs. This includes immune manipulation by the microbiota, drug-induced changes in microbial community structure and function, drug-induced changes in immune function that feedback to changes in the microbiome, and the direct microbial biotransformation of drugs, all of which could impact the activity of immunomodulatory drugs. (B) During anti-PD-1 therapy, the inhibitory signal of PD-L1 from tumor cells is blocked, allowing for enhanced anti-tumor response. However, the efficacy of anti-PD-1 therapy depends on sufficient numbers of tumor infiltrating lymphocytes (TILs). The composition of the microbiota can influence the quantity of TILs as demonstrated in recent work in which colonization with A. muciniphila increases CCR9+ CXCR3+ CD4+ TILs in an IL-12-dependent manner during anti-PD-1 treatment in mice. (C) F. prausnitzii has been associated with anti-inflammatory responses through the inhibition of NF-κB signaling via a microbial anti-inflammatory molecule. Despite this association, patients on the immunosuppressant drug tacrolimus with elevated levels of F. prausnitzii required higher drug doses because F. prausnitzii metabolizes tacrolimus into a less active metabolite (M1), providing an example of a bacterium that can have opposing impacts on therapy.