Figure 2. Cell intrinsic and extrinsic mechanisms regulating hematopoietic stem cells aging.
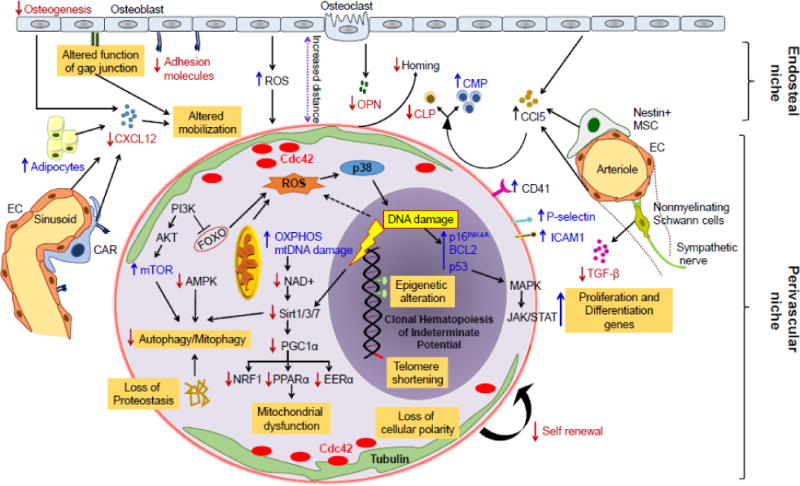
Aging negatively affects HSC function through both intrinsic and hematopoietic microenvironment mediated mechanisms. Intrinsic effects include increased ROS levels leading to DNA damage and replicative senescence, epigenomic alterations with H4K16ac, H4K20me3 and H3K4me3 and telomere attrition. Increased ROS in aged HSC consequently activates p38-MAPK, followed by induction of cell cycle regulators p53, BCL2 and p16Ink4a, resulting in an age-associated decline in HSC function and increased senescence. Moreover, aging associated amplification of nutrient sensing PI3K/Akt/mTOR pathway and the inhibition of downstream FOXO (1, 3 and 4) transcription factors, as well as metabolic alterations comprising decrement in NAD+ or Sirtuins (Sirt1/3/7) and consequent alleviation of transcription factors Nrf1, ERRα, and PPARα are involved in loss of proteostasis, autophagy/mitophagy and mitochondrial biogenesis in aged HSC. Low calorie intake however activate AMPK pathway, and promotes HSC quiescence and self-renewal. Increase in Cdc42 activity and subsequently loss of Cdc42 and tubulin polarization in aged HSC associated with defective HSC self-renewal activity. Aging associated changes in hematopoietic microenvironment including high oxidative stress, decreased adhesion to bone marrow stromal cells, increased adipogenic differentiation and decreased mesenchymal progenitors also linked with reduced HSC self-renewal and differentiation, homing as well as HSC mobilization. Various niche cells including osteoblasts, endothelial cells (EC), Nestin+ mesenchymal stem cells (MSC), CXCL12 abundant retricular (CAR) cells and nonmyelinating Schwann cells have been implicated for their roles in HSC aging. In addition, aging of HSC increased CD41 expression, which modulates HSC long-term repopulation and survival as well as myeloid skewing, while increased levels of surface adhesion molecules P-selectin (CD62P) and intercellular adhesion molecule 1 (ICAM1/CD54) have been associated with enhanced HSC mobility and attenuated engraftment. Red down arrows represent repressive and blue up arrows represents activating signals. Reactive oxygen species (ROS), osteopontin (OPN), common myeloid progenitor (CMP), common lymphoid progenitor (CLP), mitochondrial DNA (mtDNA), oxidative phosphorylation (OXPHOS), Forkhead O (FOXO), nuclear respiratory factor 1 (Nrf1), estrogen-related receptor alpha (ERRα), Peroxisome proliferator-activated receptor alpha (PPARα), CXC-chemokine ligand 12 (CXCL12), CC-chemokine ligand 5 (CCL5).
