Abstract
Background:
Infectious Diseases Society of America (IDSA) guidelines recommend empiric antipseudomonal combination therapy when Pseudomonas is suspected. However, combination antipseudomonal therapy is controversial. This study compares all-cause 30-day mortality in older patients who received antipseudomonal monotherapy (PMT) or antipseudomonal combination therapy (PCT) for the treatment of community-onset pneumonia.
Methods:
This population-based, retrospective, cohort study used data from over 150 Veteran Health Administration hospitals. Patients were classified as low, medium, or high risk of drug-resistant pathogens. 31,027 patients were assigned to PCT or PMT treatment arms based on antibiotics received in the first 48 hours of hospital admission.
Results:
The unadjusted 30-day mortality difference between PCT and PMT was most pronounced in the low-risk group (18% vs. 8%), followed by the medium-risk group (24% vs. 18%) and the high-risk group (39% vs. 33%). PCT was associated with higher 30-day mortality than PMT overall (aOR, 1.54; 95%CI, 1.43-1.66), in all three risk groups: low (aOR, 1.69; 95%CI, 1.50-1.89), medium (aOR, 1.30; 95%CI, 1.14-1.48), and high (aOR, 1.21; 95%CI, 1.04-1.40).
Conclusion:
Older adults who received combination antipseudomonal therapy for community-onset pneumonia fared worse than those who received monotherapy. Empiric combination antipseudomonal therapy should not be routinely offered to all patients suspected of having pseudomonal pneumonia.
Keywords: pneumonia, Pseudomonas, risk score, survival, mortality, antibiotics
Background
Antibiotics are the mainstay of treatment for pneumonia; however, therapy is not “one size fits all”. Furthermore, timely administration of antibiotics is critical, so initial empiric treatment, followed by pathogen-directed therapy, is required. One of the most controversial questions to date involves the choice of empiric antipseudomonal monotherapy (PMT) or antipseudomonal combination therapy (PCT) for patients suspected of having community-onset pneumonia due to Pseudomonas. Clinicians want to do what is best for their patients, including adequate coverage, to increase the probability of a positive outcome; however, they also want to limit their patients’ exposure to broad-spectrum antibiotic therapies that may result in health complications and promote antibiotic resistance. Antibiotics are a precious resource and prescribers want to be good stewards, but lack high-quality evidence to inform this important decision. The available evidence comes from small, single-center studies, and is often contradictory [1]. Clinicians are left to wonder, “Should I routinely prescribe PCT for my patients presenting with community-onset pneumonia and multidrug resistant (MDR) risk factors?”
When Pseudomonas pneumonia is suspected, American Thoracic Society / Infectious Diseases Society of America (ATS/IDSA) pneumonia guidelines recommend PCT with two or more antipseudomonal therapies, including: beta-lactam plus aminoglycoside, beta-lactam plus fluoroquinolone, or aminoglycoside plus fluoroquinolone [2]. Nevertheless, several studies have failed to observe additional benefit with PCT versus PMT in the setting of Pseudomonas pneumonia [3–7]. Early studies were promising for PCT [8], but more recent studies have not been able to reliably confirm those findings [9–11]. Additional studies have demonstrated a mortality benefit only for the sickest pneumonia patients [12], including those with Pseudomonas bacteremia or as definitive treatment [13,14].
Since clinicians rarely know if a patient has Pseudomonas bacteremia on admission, and timely empiric therapy is critical, it is helpful to use prediction rules to decide who gets any type of empiric antipseudomonal therapy (PMT or PCT). One of our prior studies confirmed that such rules can be used effectively to identify patients likely to benefit from empiric antipseudmonal therapy [15]. However, it is unclear if such rules can also be used to identify patients who might experience additional benefit from PCT (versus PMT); therefore, the objective of this study was to compare all-cause 30-day mortality in older patients who received PCT (versus PMT) for the treatment of community-onset pneumonia, stratified based on risk level of having pseudomonal pneumonia.
Methods
This population-based, retrospective, cohort study used data from over 150 hospitals and 1,400 clinics in the Veterans Health Administration (VHA) system between fiscal years 2002 and 2007. The methods used to build the database, and define the study population, have been previously published [16–19]. Briefly, data for this study were obtained from the VHA electronic medical record system that includes administrative, clinical, laboratory, and pharmacy data. These data were generated during the course of care and billing for VHA patients, and not for research; however, the study investigators used these pieces of data to construct variables that can be used for research. For instance, comorbidities were defined using International Classification of Diseases (ICD) codes. It is important to note that this was not a sample or subset of the VHA population. Rather, this was a population-based study of all patients in the VHA system. The Institutional Review Board of the University of Texas Health Science Center at San Antonio and the South Texas Veterans Health Care System Research and Development committee approved this study.
Patients were included if they were ≥ 65 years of age and had either a primary discharge diagnosis of pneumonia/influenza (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 480.0–483.99 or 485–487) or a secondary discharge diagnosis of pneumonia/influenza plus a primary diagnosis of respiratory failure (ICD-9-CM code 518.81) or sepsis (ICD-9-CM code 038.xx). Comorbid conditions were determined using ICD-9-CM codes from outpatient and inpatient care in accordance with the Charlson comorbidity scoring system [20, 21]. If patients were admitted more than once during the period of study, only the first episode qualified for inclusion in the study. Patients who did not receive antipseudomonal therapy, and those who received it after 48 hours post admission, were excluded.
The risk score tool and variables for the stratification of these patients was based on the risk score developed by Ma et al. and modified for our database, as earlier defined [17]. Briefly, the risk level groups [low (0.0-2.0), medium (2.5-7.0), and high (7.5-21.5)], were generated based on the number of points a patient had, as contributed by the total score from the comorbidities presented by the individual. A total of 31,027 patients received antipseudomonal therapy in the first 48 hours of admission and were stratified into PMT or PCT arms for each risk group. Antipseudomonal therapies were defined as the receipt of specific beta-lactams, fluoroquinolones, or aminoglycosides with in vitro activity against Pseudomonas. Table 1 depicts the complete list of all antibiotics considered in this study, and definitions of guideline-concordant community-acquired pneumonia (GC-CAP) therapy, Pseudomonas therapy, and methicillin-resistant Staphylococcus aureus (MRSA) therapy.
Table 1.
Definitions of antibiotic therapy
| Guideline-Concordant CAP therapy | ||
| Ward patients • Beta-lactam1 plus (macrolide2 or doxycycline) • Respiratory fluoroquinolone3 |
ICU patients • Beta-lactam1 plus (macrolide2 or doxycycline) • Beta-lactam1 plus respiratory fluoroquinolone3 |
|
| Pseudomonas therapy | ||
| • Antipseudomonal beta-lactam4 • Antipseudomonal fluoroquinolone5 • Aminoglycoside6 |
||
| MRSA therapy | ||
| • Vancomycin • Linezolid | ||
CAP: community-acquired pneumonia; ICU: intensive care unit
Beta-lactam includes cefotaxime, ceftriaxone, ampicillin-sulbactam, ertapenem, or aztreonam
Macrolide includes azithromycin, clarithromycin, or erythromycin
Respiratory fluoroquinolone includes moxifloxacin, levofloxacin, orgatifloxacin
Antipseudomonal beta-lactam includes cefepime, ceftazidime, imipenem-cilastatin, meropenem, piperacillin-tazobactam, ticarcillin-clavulanate, or aztreonam
Antipseudomonal fluoroquinolone includes ciprofloxacin or levofloxacin
Aminoglycoside includes gentamicin, tobramycin, or amikacin
The primary study outcome was all-cause 30-day mortality, which has been shown to be most closely associated with pneumonia-related mortality [22]. Mortality was assessed using the VHA Vital Status file, which is in close agreement (98%) with the National Death Index [23].
Statistical Analyses
30-day all-cause mortality was compared for patients who received PMT or PCT. All statistical analyses were conducted using JMP 10.0® (SAS Corp, Cary, NC). Categorical variables were compared by Fisher’s exact test or Chi-square. Continuous variables were compared using the Wilcoxon Rank Sum test (Table 2). For bivariable statistical tests, P values of ≤ 0.05 were considered to be statistically significant, and all tests were two-tailed.
Table 2.
Baseline characteristics for patients with antipseudomonal monotherapy (PMT) or antipseudomonal combination therapy (PCT)
| PMT (n=23,916) | PCT (n=7,111) | P-value | |
|---|---|---|---|
| Patient age (years), median (IQR) | 78 (72-82) | 78 (72-83) | 0.39 |
| Male, % | 98 | 99 | 0.0093 |
| Race, % | |||
| White | 83 | 80 | 0.0002 |
| Black | 12 | 13 | 0.70 |
| Other | 5 | 7 | <0.0001 |
| Hispanic ethnicity, % | 6 | 10 | <0.0001 |
| MDR risk score variables (points), % | |||
| Respiratory organ failure (14pts) | 8 | 21 | <0.0001 |
| Hospitalization in the past 90 days (5pts) | 24 | 38 | <0.0001 |
| Invasive mechanical ventilation (2pts) | 4 | 17 | <0.0001 |
| Healthcare-associated pneumonia risk factor (0.5pts) | 35 | 50 | <0.0001 |
| MDR risk score, median (IQR) | 0 (0-5.5) | 5.5 (0-5.5) | <0.0001 |
| Low (0.0-2.0), % | 70 | 48 | <0.0001 |
| Medium (2.5-7.0), % | 21 | 30 | <0.0001 |
| High (7.5-21.5), % | 9 | 22 | <0.0001 |
| Charlson comorbidity score, median (IQR) | 2 (1-4) | 3 (1-4) | <0.0001 |
| Comorbid conditions, % | |||
| Myocardial infarction | 7 | 8 | <0.0001 |
| Heart failure | 26 | 26 | 0.86 |
| Chronic obstructive pulmonary disease | 53 | 52 | 0.0097 |
| Liver disease | 1 | 1 | 0.17 |
| Renal disease | 12 | 15 | <0.0001 |
| Diabetes | 33 | 35 | 0.0039 |
| Neoplastic disease | 26 | 30 | <0.0001 |
| HIV/AIDS | <1 | <1 | 0.13 |
| Medication use within 90 days, % | |||
| Cardiovascular medications | 71 | 65 | <0.0001 |
| Anti-diabetic medications | 23 | 22 | 0.53 |
| Inhaled corticosteroids | 24 | 21 | <0.0001 |
| Systemic corticosteroidsa | 24 | 25 | 0.37 |
| Pulmonary medications | 39 | 36 | <0.0001 |
| Vasopressors, % | 3 | 12 | <0.0001 |
| Invasive mechanical ventilation, % | 4 | 17 | <0.0001 |
| Noninvasive mechanical ventilation, % | 3 | 7 | <0.0001 |
| Hemodialysis, % | 15 | 20 | <0.0001 |
| Organ failure, % | |||
| Any organ failure, % | 21 | 39 | <0.0001 |
| Respiratory | 8 | 21 | <0.0001 |
| Cardiovascular | 5 | 9 | <0.0001 |
| Neurological | 2 | 3 | <0.0001 |
| Renal | 11 | 21 | <0.0001 |
| Hematologic | 2 | 5 | <0.0001 |
| Hepatic | <1 | <1 | 0.0012 |
| Antibiotic therapy, % | |||
| Guideline-concordant CAP therapy | 85 | 67 | <0.0001 |
| MRSA therapy | 49 | 59 | <0.0001 |
| Pseudomonas diagnosis code by discharge | 1 (179) | 7 (504) | <0.0001 |
CAP: community-acquired pneumonia; HIV/AIDS: human immunodeficiency virus/acquired immunodeficiency syndrome; IQR: interquartile range; MRSA: methicillin-resistant Staphylococcus aureus; PSE: Pseudomonas
Includes oral and/or injectable corticosteroids
Potential confounding was minimized using multivariate logistic regression. Separate multivariable logistic regression models were constructed to determine whether any association existed between PMT or PCT and 30-day mortality in the overall population and additionally in each of the three risk groups. The dependent variable was 30-day mortality, and the independent variable was PMT or PCT. Covariates included all characteristics from Table 2. The independent variable and all covariates were entered into the model simultaneously. Adjusted odds ratios (aORs) and 95% confidence intervals (95%CI) were calculated; those 95%CI not crossing 1 were considered to be statistically significant.
The final list of covariates included: patient age, race, Hispanic ethnicity, myocardial infarction, heart failure, chronic obstructive pulmonary disease, liver disease, renal disease, diabetes, neoplastic disease, cardiovascular medications, anti-diabetic medications, inhaled corticosteroids, systemic corticosteroids, pulmonary medications, vasopressors, invasive and non-invasive mechanical ventilation, respiratory failure, cardiovascular failure, neurological failure, renal failure, hematological failure, hepatic failure, GC-CAP therapy, and MRSA therapy. When variables were collinear, only one of the variables was used. For instance, most patients on hemodialysis also had renal failure; therefore, renal failure was chosen as the variable for the models and the hemodialysis variable was excluded. The Charlson score and the “any organ failure” variables were excluded from the models because individual comorbidities and organ failures were already included in the models. Individual risk score variables were also excluded from the models because our study ran separate multivariable models for the three risk groups, and these individual characteristics were used to define those risk groups.
Results
Overall Population
Of the 31,027 patients who met study criteria, 23% received PCT and 77% received PMT. Patients belonged to low (59%), medium (24%), and high (18%) risk groups. One or more healthcare-associated pneumonia (HCAP) criteria was the most common Multi Drug Resistant (MDR) risk score variable, followed by hospitalization in the past 90 days, respiratory organ failure, and invasive mechanical ventilation.
The median (interquartile range) Charlson score was 2 (1-4) and common comorbidities included chronic obstructive pulmonary disease (53%), diabetes (33%), heart failure (26%), and neoplastic disease (26%). The most common medications used within 90 days prior to admission were cardiovascular (71%) and pulmonary (39%) medications. Organ failure occurred in 24% of patients. Finally, 80% of patients received GC-CAP therapy and 34% received MRSA therapy.
Baseline Characteristics
Patient age (median of 78 years), race (>80% white), and sex (>98% male) were similar for patients receiving PCT or PMT (Table 2); however, many comorbid conditions including diabetes, renal disease, and heart failure were more prevalent in the PCT arm. All the MDR risk score variables were more prevalent in the PCT arm, and a greater proportion of patients in the PCT arm had organ failure.
Of note, 70% of those on PMT were classified as low-risk, compared to 50% of those on PCT. Conversely, 22% of those on PCT were high-risk, as compared to 9% of those on PMT. Finally, PMT patients were more likely to have received guideline-concordant therapy, whereas PCT patients were more likely to have received MRSA therapy and to have had a positive Pseudomonas culture by discharge.
All-cause Mortality
30-day mortality was 18% overall and increased among the risk groups: low (13%), medium (21%), and high (36%). The unadjusted mortality difference between PCT and PMT was most pronounced in the low-risk group (18% vs. 8%, 10% absolute risk difference), followed by the medium-risk group (24% vs. 18%, 6% difference) and the high-risk group (39% vs. 33%, 6% difference) (Figure 1). PCT was associated with higher 30-day mortality than PMT overall (aOR, 1.54; 95%CI, 1.43-1.66), and in all three risk groups: low (aOR, 1.69; 95%CI, 1.50-1.89), medium (aOR, 1.30; 95%CI, 1.14-1.48), and high (aOR, 1.21; 95%CI, 1.04-1.40).
Figure 1.
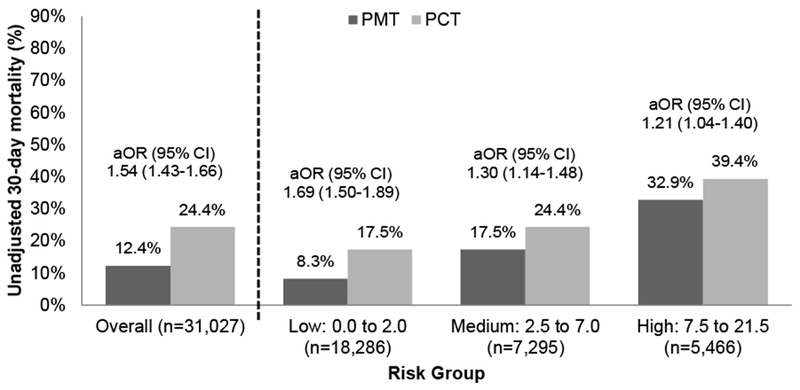
Comparison of 30-day mortality in patients who received antipseudomonal monotherapy (PMT) and antipseudomonal combination therapy (PCT)
aOR: adjusted odds ratio; 95% CI: 95% confidence interval
Discussion
The present study set out to determine if PCT (versus PMT) is associated with additional survival benefit in the high-risk group. Unfortunately, the present study found no additional benefit with PCT in any of the risk groups, including the high-risk group. Our results favored PMT significantly, with 33% mortality in the monotherapy arm versus 39% in the combination therapy arm (aOR 1.21, 95% CI 1.04 – 1.40).
Other studies have also demonstrated a lack of additional benefit with combination therapy versus monotherapy in community-onset pneumonia and other infections [9, 11, 24]. A 2004 systematic review evaluating beta-lactam monotherapy versus beta-lactam plus aminoglycoside combination therapy for sepsis found no advantage to combination therapy among patients with gram-negative infections including Pseudomonas aeruginosa infections [9]. The authors reported that patients with pneumonia had significantly fewer failures with monotherapy and there was no advantage to combination therapy. In another study, combination therapy was not associated with improved survival in patients with Gram-negative bacteremia (odds ratio, 0.96; 95%CI, 0 .70-1.32), [11] possibly due to inadequate sample size. In another study, hospital mortality was numerically higher for PCT patients (37%) compared with PMT patients (29%) (p=0.17) [24]. Though not statistically significant, the findings suggest that PCT might have been associated with poorer outcome than PMT had the study authors included a larger sample size. Several additional studies have reported no survival benefit for empiric PCT versus PMT [25–28]. The mortality rates in our study are greater than most of the prior studies, likely because our population is older with a greater proportion of patients who are male and have serious comorbidities. Nevertheless, the association observed in our study is consistent with that observed in prior studies. Furthermore, because of our large sample size, we are able to not only observe the same association (i.e., numerically better survival with PMT vs. PCT), but also observe a statistically significant difference, in favor of PMT over PCT. While our study is not the first to demonstrate this lack of additional benefit with PCT versus PMT, it is the first to do so among high-risk patients—the group that is considered to most likely benefit from PCT, and a group that has previously been shown to benefit from antipseudomonal therapy in general [17].
Our study findings may surprise some readers. After all, the current guidelines for the management of patients with community-acquired pneumonia recommend PCT for suspected pseudomonal pneumonia [2]. In light of new evidence, from this study, and our prior study [17], we believe the community-acquired pneumonia guidelines should be changed. We support the use of empiric PMT in high-risk patients, but we do not support the use of empiric PCT in pneumonia patients from any of the risk groups. PCT may be beneficial over PMT for patients with known Pseudomonas (i.e., definitive therapy)—a question beyond the scope of this study—but PCT is not associated with additional benefit in patients simply suspected of having Pseudomonas (i.e., empiric therapy).
Limitations
Despite the many strengths of our study, including its large sample size, risk stratification, and robust statistical methodologies to deal with dissimilar baseline characteristics, our study has limitations, most of which are inherent to all retrospective, observational studies. For instance, this study design can only identify an association, but not prove causation. It is possible that some unmeasured variable accounts for the lack of additional benefit with PCT. Given the retrospective nature of the study, we cannot ascertain the providers’ rationale for their prescribing practices. The study only included patients who received antibiotics within 48 hours of admission. Since it generally takes longer than 48 hours for culture and sensitivity results to return, we can presume this is empiric therapy, but we do not know that for sure. In addition, we acknowledge that several variables, which have been previously associated with greater disease severity in other pneumonia studies, were more prevalent among patients who received PCT. That is why we stratified patients into three risk groups and used multivariable regression models to mitigate baseline differences between groups. Risk stratification is a practical strategy, and warranted, given that we saw a benefit with antipseudomonal therapy in the high-risk group in our prior study. Regression modeling has been shown to adequately adjust for confounders in observational studies and provides adequate control for these confounders when estimating the effect of treatment on outcomes [29].
Conclusion
Older adults who received combination antipseudomonal therapy for community-onset pneumonia fared worse than those who received monotherapy. Combination therapy may be even more detrimental for low-risk patients than for medium- or high-risk patients. Empiric combination antipseudomonal therapy should not be routinely offered to all patients suspected of having Pseudomonas aeruginosa pneumonia.
Table 3.
Multivariable models to identify risk factors for 30-day mortality, including antipseudomonal monotherapy (PMT) or antipseudomonal combination therapy (PCT)
| Adjusted Odds ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Risk score | All (n=31,027) | 0.0-2.0 (Low Risk) (n=18,286) | 2.5-7.0 (Medium Risk) (n=7,295) | 7.5-21.5 (High Risk) (n=5,466) |
| PCT vs. PMT | 1.54 (1.43-1.66) | 1.69 (1.50-1.89) | 1.30 (1.14-1.48) | 1.21 (1.04-1.40) |
| Age (1-year increments) | 0.91 (0.90-0.92) | 0.89 (0.88-0.91) | 0.92 (0.90-0.94) | 0.92 (0.90-0.94) |
| Race | 0.99 (0.90-1.10) | 0.93 (0.80-1.08) | 1.09 (0.92-1.30) | 0.94 (0.75-1.16) |
| Hispanic ethnicity | 0.97 (0.85-1.10) | 1.06 (0.88-1.27) | 0.80 (0.63-0.99) | 1.04 (0.79-1.36) |
| Comorbid conditions | ||||
| Myocardial infarction | 1.13 (1.00-1.29) | 1.12 (0.89-1.40) | 1.02 (0.84-1.23) | 0.99 (0.76-1.29) |
| Heart failure | 1.05 (0.97-1.14) | 0.98 (0.87-1.12) | 0.99 (0.87-1.14) | 1.00 (0.85-1.18) |
| COPD | 0.95 (0.88-1.03) | 0.95 (0.84-1.06) | 0.92 (0.80-1.06) | 0.92 (0.78-1.09) |
| Liver disease | 1.32 (0.97-1.80) | 0.88 (0.46-1.54) | 1.20 (0.72-1.92) | 1.86 (1.03-3.37) |
| Renal disease | 0.94 (0.85-1.05) | 0.89 (0.75-1.05) | 0.90 (0.76-1.06) | 1.01 (0.82-1.25) |
| Diabetes | 1.03 (0.93-1.14) | 1.06 (0.91-1.23) | 0.98 (0.82-1.17) | 1.04 (0.84-1.27) |
| Neoplastic disease | 1.64 (1.52-1.76) | 1.50 (1.35-1.67) | 1.79 (1.58-2.03) | 1.42 (1.21-1.67) |
| Medication use, by class | ||||
| Cardiovascular medications | 0.74 (0.68-0.79) | 0.68 (0.61-0.76) | 0.71 (0.61-0.81) | 0.88 (0.75-1.03) |
| Anti-diabetic medications | 0.90 (0.80-1.01) | 0.78 (0.65-0.93) | 1.03 (0.84-1.26) | 0.96 (0.76-1.22) |
| Inhaled corticosteroids | 0.71 (0.65-0.79) | 0.67 (0.57-0.78) | 0.69 (0.58-0.82) | 0.88 (0.72-1.07) |
| Systemic corticosteroids | 1.11 (1.02-1.20) | 1.07 (0.94-1.23) | 1.05 (0.91-1.22) | 1.00 (0.83-1.19) |
| Pulmonary medications | 0.99 (0.91-1.09) | 0.99 (0.87-1.14) | 1.00 (0.85-1.17) | 0.91 (0.76-1.09) |
| Vasopressors | 1.66 (1.45-1.91) | 2.37 (1.81-3.09) | 2.02 (1.46-2.81) | 1.30 (1.08-1.57) |
| Mechanical ventilation | ||||
| Invasive | 0.88 (0.77-1.01) | 2.71 (2.01-3.66) | 0.85 (0.44-1.58) | 0.73 (0.62-1.34) |
| Non-invasive | 1.47 (1.28-1.70) | 2.26 (1.73-2.91) | 1.62 (1.13-2.29) | 1.11 (0.92-0.86) |
| Organ failure | ||||
| Respiratory | 2.55 (2.29-2.84) | N/A | N/A | 0.86 (0.59-1.25) |
| Cardiovascular | 1.47 (1.30-1.66) | 1.42 (1.15-1.72) | 1.28 (1.00-1.62) | 1.69 (1.39-2.07) |
| Neurological | 1.36 (1.12-1.65) | 1.30 (0.95-1.76) | 1.47 (1.01-2.11) | 1.24 (0.87-1.74) |
| Renal | 1.58 (1.45-1.73) | 1.88 (1.65-2.15) | 1.42 (1.20-1.68) | 1.33 (1.12-1.57) |
| Hematologic | 1.58 (1.34-1.86) | 1.44 (1.11-1.86) | 1.40 (1.01-1.92) | 2.00 (1.48-2.72) |
| Hepatic | 2.55 (1.51-4.30) | 2.92 (1.23-6.59) | 1.97 (0.69-5.22) | 2.78 (1.09-7.67) |
| Antibiotic therapy | ||||
| GC-CAP therapy | 0.53 (0.49-0.57) | 0.49 (0.44-0.56) | 0.59 (0.52-0.67) | 0.78 (0.67-0.90) |
| MRSA therapy | 1.22 (1.14-1.31) | 1.22 (1.10-1.34) | 1.19 (1.05-1.34) | 1.06 (0.90-1.25) |
| Pseudomonas diagnosis code by discharge | 0.66 (0.53-0.82) | 0.71 (0.47-1.04) | 0.65 (0.42-0.98) | 0.65 (0.46-0.90) |
Odds ratios greater than one indicate an increased risk of 30-day mortality, whereas odds ratios less than one indicate a decreased risk of 30-day mortality. Race was ordered as black versus nonblack. COPD: chronic obstructive pulmonary disease; GC-CAP: guideline-concordant community-acquired pneumonia.
Highlights.
Retrospective cohort study of patients with community-onset pneumonia
Antipseudomonal combination therapy has a higher mortality rate than monotherapy
Antipseudomonal combination therapy should not be routinely offered to all patients
Acknowledgements
The database was built with grant support from the National Institutes of Health (NIH) / National Institute of Nursing Research (R01 NR010828) to Dr. Mortensen. Dr. Frei was supported in part by a NIH Clinical Research Scholar (KL2) career development award (National Center for Research Resources KL2 RR025766 and National Center for Advancing Translational Sciences KL2 TR000118) and a NIH Clinical and Translational Science Award (UL1 TR002645 and TL1 TR002647) during part of the time during which this study was conducted. Dr. Mortensen was supported in part by a grant from the Agency for Healthcare Research and Quality (R24 HS022418) and the University of Texas Southwestern Center for Patient-Centered Outcomes Research. This material is the result of work supported with resources and the use of facilities at the South Texas Veterans Health Care System. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health.
Competing interests
Dr. Frei has received research grants, to his institution, for investigator-initiated cancer and infectious diseases research, from Allergan (formerly Forest) and AstraZeneca in the past three years.
List of abbreviations
- aOR:
adjusted odds ratio
- CAP:
community-acquired pneumonia
- COP:
community-onset pneumonia
- CI:
confidence interval
- COPD:
chronic obstructive pulmonary disease
- GC:
guideline-concordant
- HCAP:
healthcare-associated pneumonia
- ICD-9-CM:
International Classification of Diseases, Ninth Revision, Clinical Modification codes
- ICU:
intensive care unit
- IV:
intravenous
- IQR:
interquartile range
- MDR:
multidrug-resistant
- MRSA:
methicillin-resistant Staphylococcus aureus
- PSE:
Pseudomonas
- VHA:
Veterans Health Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tang SY, Zhang SW, Wu JD, et al. Comparison of mono- and combination antibiotic therapy for the treatment of Pseudomonas aeruginosa bacteraemia: A cumulative meta-analysis of cohort studies. Exp Ther Med 2018;15:2418–28. (PMC5795571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med 1989;87:540–6. [DOI] [PubMed] [Google Scholar]
- 4.Leibovici L, Paul M, Poznanski O, et al. Monotherapy versus beta-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob Agents Chemother 1997;41:1127–33. (PMC163862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamma PD, Turnbull AE, Harris AD, Milstone AM, Hsu AJ, Cosgrove SE. Less is more: combination antibiotic therapy for the treatment of gram-negative bacteremia in pediatric patients. JAMA Pediatr 2013;167:903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul M, Leibovici L. Editorial commentary: combination therapy for Pseudomonas aeruginosa bacteremia: where do we stand? Clin Infect Dis 2013;57:217–20. [DOI] [PubMed] [Google Scholar]
- 7.Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med 2015;372:1312–23. [DOI] [PubMed] [Google Scholar]
- 8.Anderson ET, Young LS, Hewitt WL. Antimicrobial synergism in the therapy of gram-negative rod bacteremia. Chemotherapy 1978;24:45–54. [DOI] [PubMed] [Google Scholar]
- 9.Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ 2004;328:668 (PMC381218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyland DK, Dodek P, Muscedere J, Day A, Cook D, Canadian Critical Care Trials G. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med 2008;36:737–44. [DOI] [PubMed] [Google Scholar]
- 11.Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis 2004;4:519–27. [DOI] [PubMed] [Google Scholar]
- 12.Traugott KA, Echevarria K, Maxwell P, Green K, Lewis JS 2nd. Monotherapy or combination therapy? The Pseudomonas aeruginosa conundrum. Pharmacotherapy 2011;31:598–608. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Park HJ, Moon SM, et al. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeruginosa pneumonia. BMC Infect Dis 2012;12:308 (PMC3519646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 2003;47:2756–64. (PMC182644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teshome BF, Lee GC, Reveles KR, et al. Application of a methicillin-resistant Staphylococcus aureus risk score for community-onset pneumonia patients and outcomes with initial treatment. BMC Infect Dis 2015;15:380 (PMC4575496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J 2011;38:878–87. [DOI] [PubMed] [Google Scholar]
- 17.Frei CR, Rehani S, Lee GC, et al. Application of a risk score to identify older adults with community-onset pneumonia most likely to benefit from empiric Pseudomonas therapy. Pharmacotherapy 2017;37:195–203. (PMC5310964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortensen EM, Halm EA, Pugh MJ, et al. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA 2014;311:2199–208. (PMC4109266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortensen EM, Nakashima B, Cornell J, et al. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis 2012;55:1466–73. (PMC3491858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 22.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:2 (PMC1458356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher SG, Weber L, Goldberg J, Davis F. Mortality ascertainment in the veteran population: alternatives to the National Death Index. Am J Epidemiol 1995;141:242–50. [DOI] [PubMed] [Google Scholar]
- 24.Bowers DR, Liew YX, Lye DC, Kwa AL, Hsu LY, Tam VH. Outcomes of appropriate empiric combination versus monotherapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 2013;57:1270–4. (PMC3591924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulsson M, Granrot A, Ahl J, et al. Antimicrobial combination treatment including ciprofloxacin decreased the mortality rate of Pseudomonas aeruginosa bacteraemia: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2017;36:1187–96. (PMC5495847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deconinck L, Meybeck A, Patoz P, et al. Impact of combination therapy and early deescalation on outcome of ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Infect Dis (Lond) 2017;49:396–404. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, Jun YH, Kim YR, et al. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; retrospective study of impact of combination antimicrobial therapy. BMC Infect Dis 2014;14:161 (PMC3994322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon YK, Kim HA, Ryu SY, et al. Tree-structured survival analysis of patients with Pseudomonas aeruginosa bacteremia: a multicenter observational cohort study. Diagn Microbiol Infect Dis 2017;87:180–7. [DOI] [PubMed] [Google Scholar]
- 29.Spiegelman D, Zhou X. Evaluating public health interventions: 8. causal inference for time-invariant interventions. Am J Public Health 2018;108:1187–90. (PMC6085031) [DOI] [PMC free article] [PubMed] [Google Scholar]


