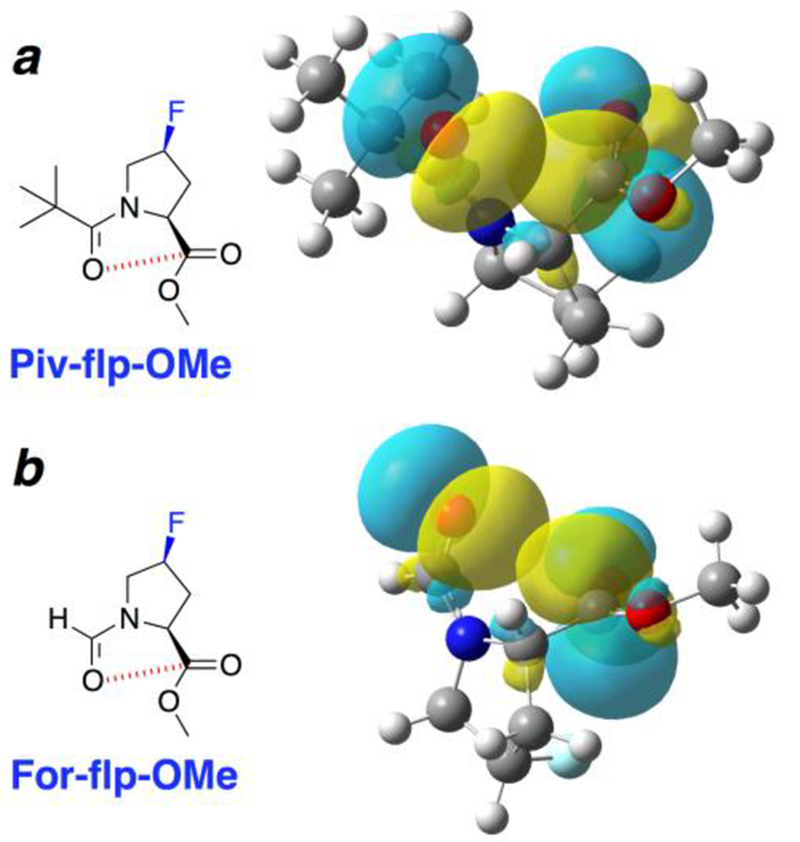
Figure 5.
Natural bond orbital (NBO) analysis[25] of geometry-optimized structures of Piv-flp-OMe and For-flp-OMe, with a trans amide bond, endo ring pucker, and PPII/β conformation. By this analysis, the conformation in the pivaloyl derivative is stabilized by 0.52 kcal mol–1 by the n→π* interaction, while in the formyl derivative the conformation is only stabilized by 0.12 kcal mol–1. These energy differences are reflected in the differing extents of orbital overlap and the divergent Oi…Ci+1 intercarbonyl distances (Piv: d = 2.92 Å; For: d = 3.17 Å). Additional computational analysis as a function of acyl capping group and conformation is included in the Supporting Information.