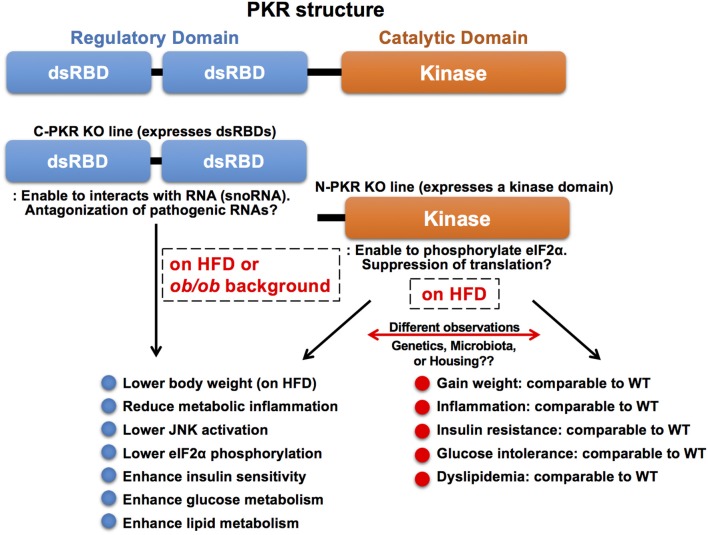
Figure 4.
Molecular structure and knockout mouse models of PKR. PKR is a serine/threonine kinase composed of a N-terminal regulatory domain containing two dsRBDs and a C-terminal catalytic (kinase) domain. The two dsRBDs bind to dsRNA leading to conformational change and kinase activation. There are two PKR-deficient mouse models used in the study of PKR function. Both PKR-deficient mouse models are incomplete knockouts and retain partial functionality of PKR. The N-PKR KO model lacks two dsRBDs, but expresses a kinase domain that enables the phosphorylation of eIF2α. Conversely, the C-PKR KO model lacks the kinase domain, but expresses two dsRBDs. C-PKR KO mice showed beneficial metabolic phenotypes in the pathogenesis of obesity, however, there were contradictory findings in metabolic phenotypes observed in N-PKR KO mice. The differences may be due to environmental, genetic, and/or epigenetic factors that arise within different N-PKR KO mouse colonies.