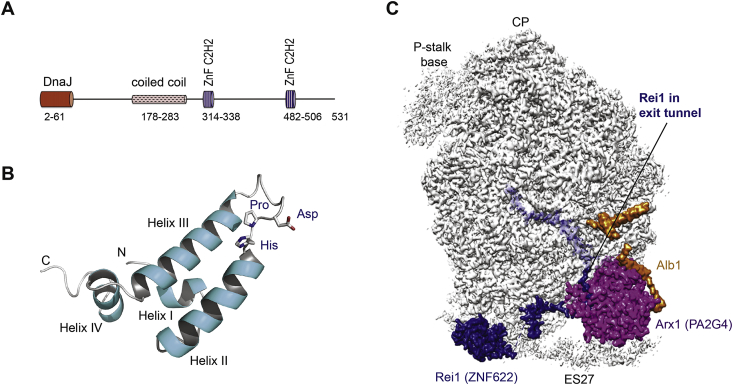
Fig. 4.
The J-protein DNAJC21 facilitates release of the export receptor Arx1 (human PA2G4) from the pre-60S ribosomal subunit. (A) Schematic representation of the domain structure of human DNAJC21 (NP_001335349). ZnF, zinc finger. (B) Ribbon representation of the NMR structure of the J-domain (residues 2–108) from E. coli DnaJ protein (pdb 1xbl) (Pellecchia et al., 1996). J-domains comprise four α-helices. The invariant His, Pro, Asp (HPD) tripeptide in the loop between helices II and III is critical to stimulate the ATPase activity and in vivo function of its cognate cochaperone heat shock 70 kDa protein. (C) Visualising the 60S-bound assembly factors Arx1 (human PA2G4), Rei1 (human ZNF622) and Alb1 near the polypeptide exit tunnel (pdb 5APO) (Greber et al., 2012). The Arx1 protein shields the polypeptide exit tunnel, which is deeply probed by the C-terminus of the Rei1 protein. Alb1 likely modulates the affinity of Arx1 binding to the 60S subunit.