Figure 1.
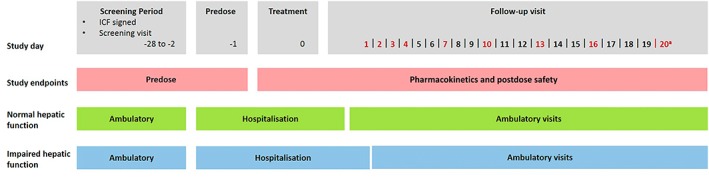
Study design overview. aLast follow‐up visit. ICF, informed consent form. Red text denotes the days of the follow‐up visits. Participants with impaired hepatic function were hospitalized for two days after treatment, controls for one day after treatment
